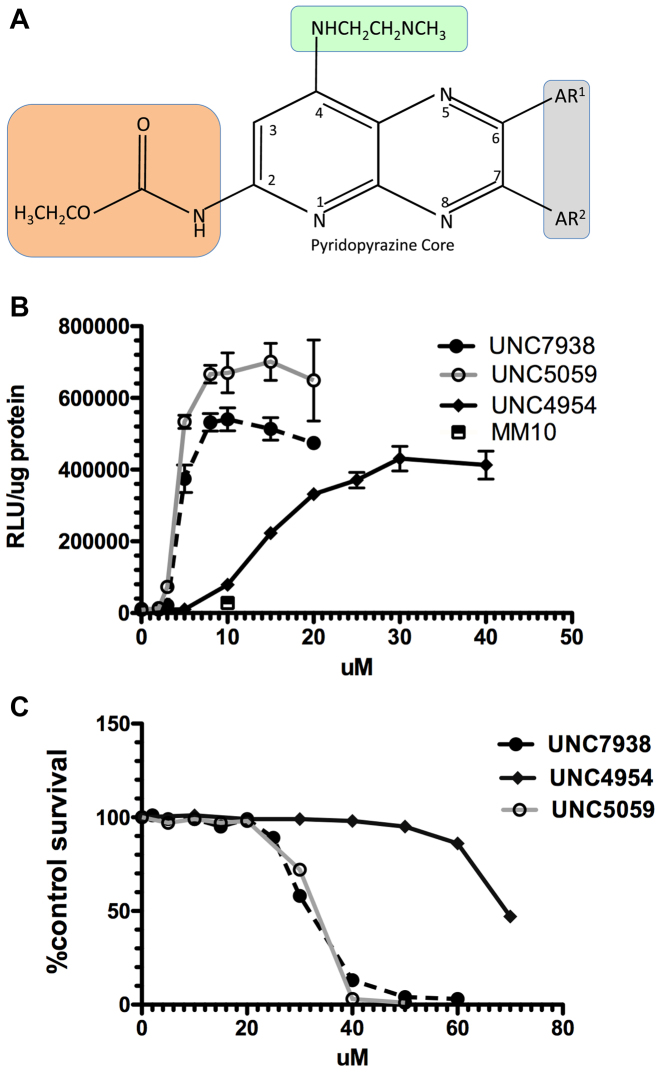
Figure 1.
(A) Structure of UNC7938 Analogs. The structure of UNC7938 and the various groups that were modified in the analogs synthesized in this study are depicted. AR1 and AR2 are aromatic groups (gray shaded area). The orange shaded area is the carbonate group. The green shaded area is the tertiary amine group. Detailed structures of analogs are shown in Supplementary Tables S1–S4. (B). OECs Enhance Oligonucleotide Effects. HeLa Luc 705 cells were incubated in 24-well plates (50,000 per well) with 100 nM SSO623 or its mismatched control (MM) for 16 h in DMEM + 10% FBS, rinsed and then treated with various concentrations of OEC compounds for 2 h. The cells were then rinsed and incubation continued for an additional 4 h in DMEM + 10% FBS. Cells were rinsed twice in PBS and luciferase activity (RLU) and cell protein determined. The square symbol indicates the MM oligonucleotide with UNC7938. Means ± SE. N = 3. (C) Cytotoxicity of OECs. HeLa Luc705 cells were exposed to compounds as in (B) then incubated for 24 h in DMEM + 10% FBS and tested using the Alamar Blue cytotoxicity assay. Means ± SE. N = 3.