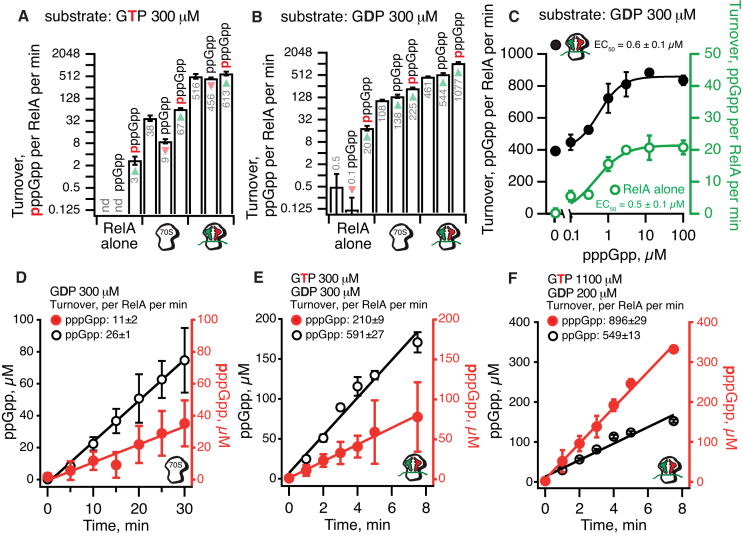
Figure 2.
Allosteric regulator pppGpp and substrate GDP synergize for maximum enzymatic activity of E. coli RelA. Enzymatic activity of 30 nM RelA assayed in the presence of 1 mM (A-C) or 4 mM (D-F) ATP and 0.3 mM of either 3H-labelled GTP (A) or GDP (B and C); in the presence of equal amounts of GDP and GTP (0.3 mM each) (D and E); or in the presence of 0.2 mM GDP and 1.1 mM GTP (F). In the case of D–F both 3H-ppGpp and 3H-pppGpp were synthesized in the reaction. As indicated on the figure, the reaction mixtures were supplemented with combinations of 0.5 μM vacant 70S or ‘starved’ ribosomal complexes programmed with 2 μM model mRNA(MF) encoding Met-Phe and deacylated tRNAiMet (2 μM; P-site) and tRNAPhe (2 μM; A-site) as well as ppGpp or pppGpp added either 100 μM (A and B) or varying concentrations (C). All experiments were performed in HEPES:Polymix buffer, pH 7.5 at 37°C in the presence of 5 mM Mg2+. Error bars represent SDs of the turnover estimates by linear regression and each experiment was performed at least three times.