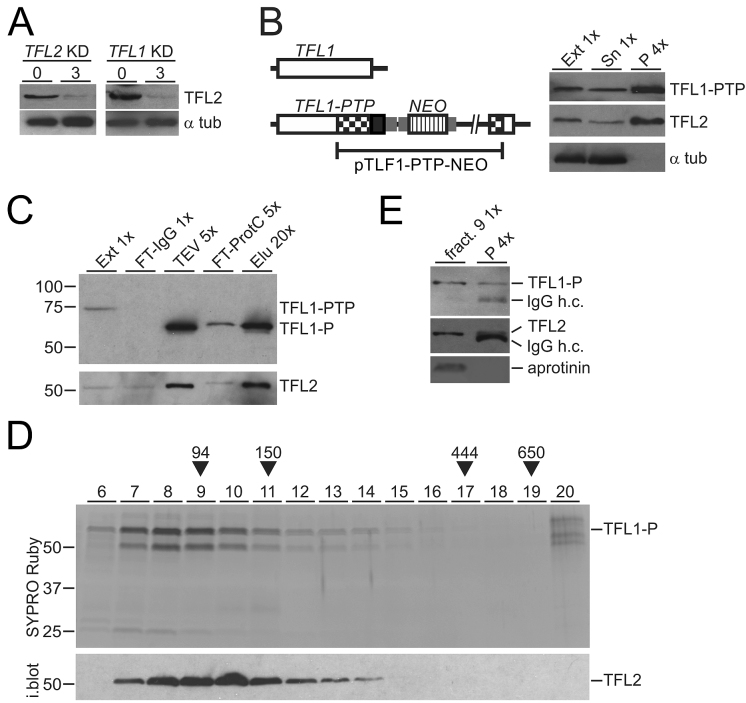
Figure 4.
TFL1 and TFL2 form a complex. (A) Lysates of uninduced cells and of cells in which TFL2 or TFL1 was knocked down (KD) for 3 days were immunoblotted and probed with anti-TFL2 immune serum and, as a loading control, with an anti-α tubulin (α tub) antibody. (B) Schematic on the left shows integration of plasmid TFL1-PTP-NEO into the TFL1 locus which fused the PTP tag sequence to the 3′ end of the TFL1 coding region. The immunoblot on the right shows TFL1-PTP, TFL2 and α tubulin in extract (Ext), supernatant (Sn) and precipitate of a TFL1-PTP pull-down with IgG beads. x-Values specify relative amounts loaded. (C) Immunoblot monitoring of TFL1-PTP tandem affinity purification with the HPC4 antibody analogous to RPB9-PTP purification of Figure 1B. Detection of TFL2 on the same blot showed efficient co-purification of this protein. (D) The final eluate of a TFL1-PTP tandem affinity purification was sedimented through a 10–40% linear sucrose gradient by ultracentrifugation, and the gradient fractionated into 20 aliquots from top to bottom. Proteins from fractions 6–20 were separated by SDS-PAGE and stained with SYPRO Ruby. As sedimentation markers with known molecular masses, Taq DNA polymerase (94 kDa), mouse IgG (150 kDa, 6.6S), apoferritin (444 kDa, 17S), and thyroglobulin (650 kDa, 19S) were co-analyzed. After gel destaining, proteins were immunoblotted and probed with anti-TFL2 immune serum (i. blot.). (E) TFL2 was immunoprecipitated from fraction 9 of the sucrose gradient and detected with anti-TFL2 immune serum. Coprecipitation of TFL1-P was analyzed on the same blot with anti-ProtC antibody. Coomassie blue-stained aprotinin which was added to fraction 9 prior to precipitation served as a negative control. In both immunoblot panels, co-eluted IgG heavy chain (IgG h.c.) was detected.