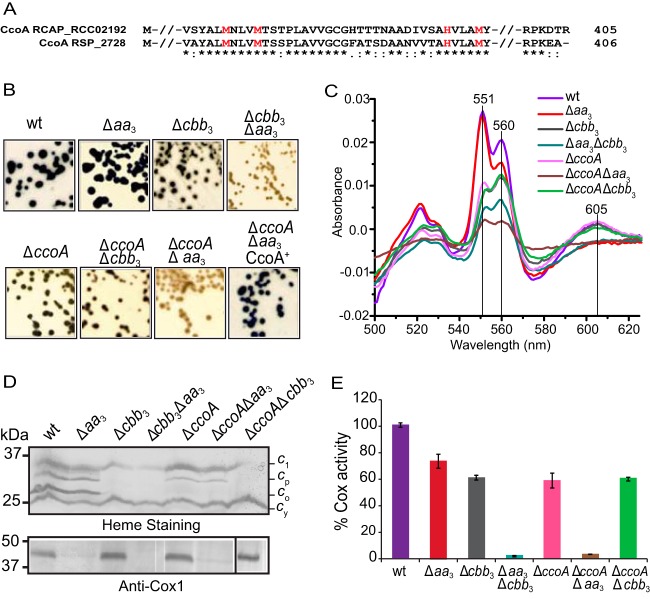
FIG 3 .
Met motifs of CcoA and phenotypic and functional characterization of R. sphaeroides ΔccoA mutants. (A) Amino acid sequence alignment of the highly conserved region surrounding the Met and His motifs MXXXM and HXXXM (in red) from R. capsulatus CcoA (RCA_RCC02192) and its R. sphaeroides homologue (RSP_2726) (65% identity; 80% similarity). (B) Growth and NADI phenotypes of colonies of R. sphaeroides wild-type (wt) Ga and Δaa3 (JS100), Δcbb3 (MT001), and Δaa3 Δcbb3 (ME127) Cox mutants together with those of ΔccoA (HW3), ΔccoA Δcbb3 (HW24), and ΔccoA Δaa3 (HW2) CcoA mutants. Cells were grown aerobically at 35°C on LB medium, and the presence of Cox activity was visualized by NADI staining (see Materials and Methods). Colonies that contain wild-type levels of Cox activity turn dark blue within a few seconds (NADI+), while those that have low or no Cox activity show lighter blue (NADIslow) or no blue staining (NADI−) upon longer exposure, respectively. Note that the ΔccoA Δaa3 mutant is NADI– like the Δaa3 Δcbb3 mutant, unless it is complemented with a plasmid carrying a wild-type allele of ccoA (ΔccoA Δaa3 CcoA+). (C) Absorption difference spectra of membrane fractions of R. sphaeroides mutants recorded between 500 and 625 nm by using oxidized membrane preparations as the baseline and reducing the sample with an excess of sodium dithionite. The intensity of the peaks centered at 551, 560, and 605 nm indicates the contents of c-, b- and a-type hemes, respectively. (D) Steady-state levels of structural subunits of cbb3- and aa3-Cox enzymes in the membranes of R. sphaeroides mutants. (Top) Membrane preparations of R. sphaeroides mutants separated by SDS-PAGE and then stained with TMBZ. Four different cyts c (cyt c1 of the cyt bc1 complex, cyt cp [CcoP] and co [CcoO] subunits of cbb3-Cox, and the membrane-attached electron carrier cyt cy) can be seen in the wild-type strain (39). In the ΔccoA mutant, the steady-state levels of cyt cp and especially cyt co are very low. (Bottom) Membrane preparations of R. sphaeroides strains resolved by SDS-PAGE and subjected to immunoblot analysis. The presence of the Cox1 subunit of R. sphaeroides aa3-Cox was identified with P. denitrificans Cox1 polyclonal antibodies that cross-react with it. The white lines seen on the blot next to some lanes are scanning artifacts and do not reflect spliced gels. (E) cyt c activity of membrane fractions of R. sphaeroides ΔccoA mutants. Total Cox (cbb3-Cox plus aa3-Cox) activities were determined using membrane preparations of various R. sphaeroides strains by monitoring the rate of oxidation of reduced horse heart cyt c. R. sphaeroides wild-type strain Ga exhibited an activity of ~1.33 µmol of cyt c oxidized/min/mg of total membrane proteins, which was referred to as 100%. Three independent assays were carried out for each strain. The ΔccoA Δaa3 mutant has no activity, like the Δaa3 Δcbb3 mutant that lacks both Cox enzymes.