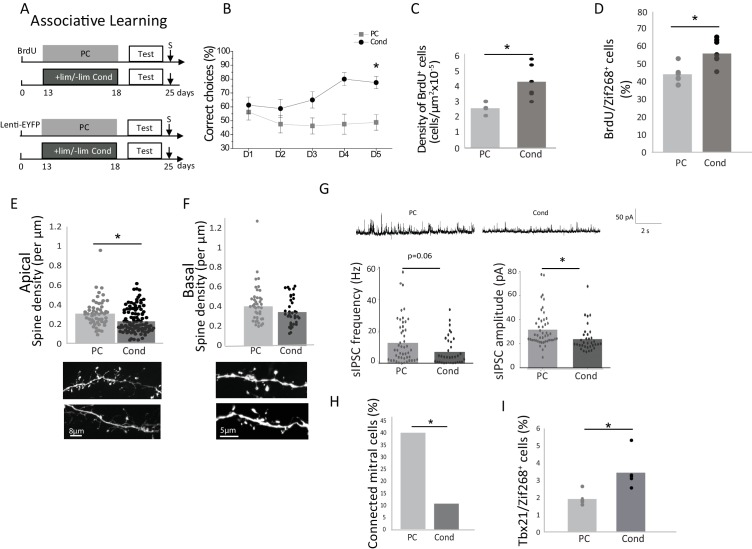
Figure 2. Behavioral and neural effects of explicit learning.
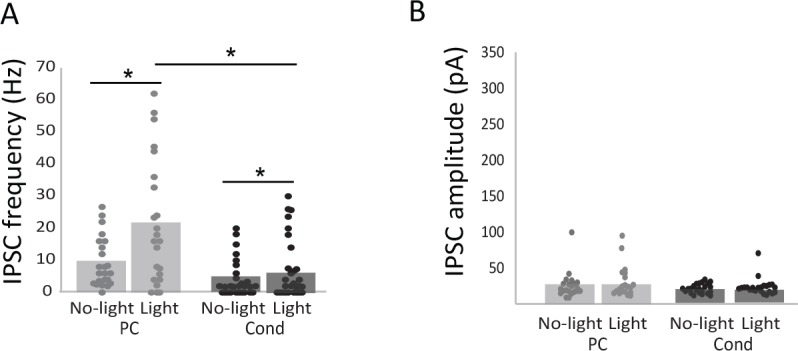
(A) Experimental design for explicit learning (S: Sacrifice, Test:Habituation/Cross Habituation Task). (B) From D1 to D5 of training, the percentage of correct choices increased in conditioned (Cond) but not pseudo-conditioned animals (PC) indicating that learning occurred only in the conditioned animals (C) Adult-born cell (BrdU-positive cell) density is increased after explicit learning (D) The percentage of odor-responsive adult-born cells (expressing Zif268) is increased after explicit learning. (E). Spine density of the apical domain decreased after explicit learning. (F) Spine density of the basal domain is unchanged after explicit learning. (G) Representative traces of sIPSC for Cond and PC (up). sIPSC frequency and amplitude are decreased after explicit learning (down). (H) Percentage of mitral cells exhibiting a significant response to light stimulation of adult-born granule cells. (I) The percentage of mitral cells (Tbx21) expressing Zif268 is higher in Cond versus PC animals. *p<0.05; the data are expressed as mean values ± SEM.
Figure 2—figure supplement 1. Improvement in discrimination after explicit learning.
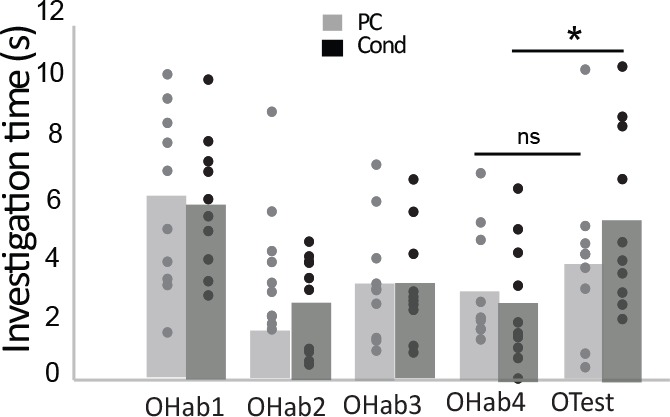
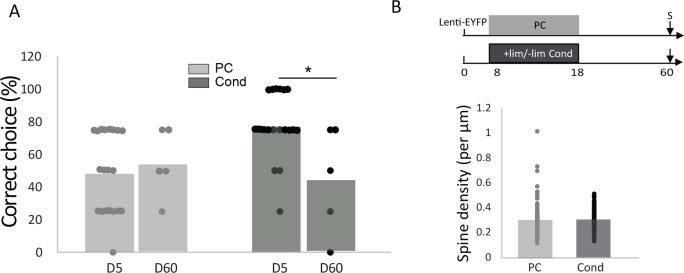
Figure 2—figure supplement 2. Long-term delay after explicit learning A.
Figure 2—figure supplement 3. Effect of light stimulation of adult-born neuron on mitral cell activity.