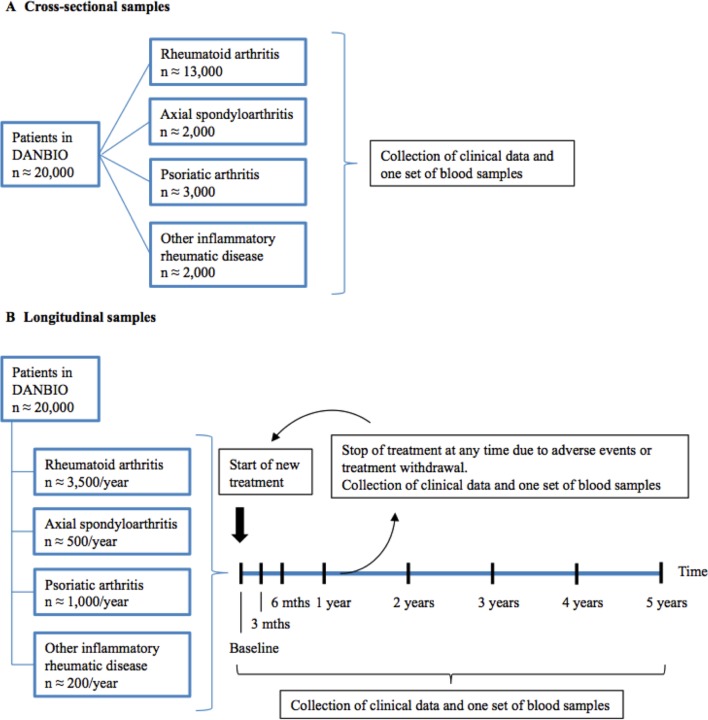
Figure 1.
Schematic presentation of study design and sampling strategies. Any patient diagnosed with RA, AxSpA, PsA, other inflammatory rheumatic disease or tissue disorder, or suspected for one of these, may participate when they meet for a scheduled routine clinical visit. These patients can provide one cross-sectional blood sample (A) or may be included for longitudinal follow-up (B) when they start treatment with a new DMARD (see text). See figure 2 for details on blood handling and storage. Numbers (n) indicate patients potentially eligible for inclusion in one or more of the study arms at the time of protocol approval. By 1 January 2018, the number of patients in DANBIO is approximately 50,000. AxSpA, axial spondyloarthritis; DMARD, disease-modifying antirheumatic drug; PsA, psoriatic arthritis; RA, rheumatoid arthritis.