FIG. 3.
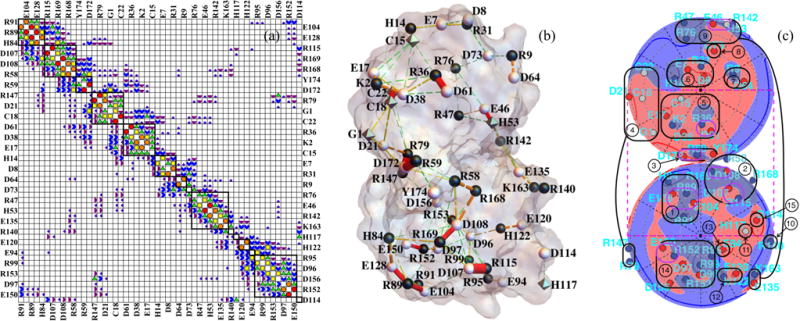
(a) Approximate block-diagonal form of the dimensionless work-of-charging matrix W for interior dielectric coefficient 3 and Debye length 6.0 Å. The Wij magnitude categories are as follows: white < 0.05 ≤ dark purple quarter circles < 0.1 ≤ purple half circles < 0.2 ≤ blue 3/4 circles < 0.4 ≤ green triangles < 0.8 ≤ yellow squares < 1.6 ≤ orange pentagons < 3.2 ≤ red circles. The matrix includes all 54 titratable residues used in the present model, which as noted in the text omits the tyrosine residues and four of the cysteine residues; the entire protein contains 174 residues. Designations for the 54 residues considered alternate between left and right (and top and bottom) margins. (b) Cylinders with radii proportional to Wij mapped onto the PDB 1AMM structure of γ B-crystallin. The Wij magnitude categories are as follows: 0.4 ≤ green cylinders with three gaps < 0.8 ≤ yellow cylinders with two gaps < 1.6 ≤ orange cylinders with one gap < 3.2 ≤ red cylinders. (c) Lambert projections with potential and charges indicated as in Fig. 1(c). Groups of titratable sites participating in approximate blocks of W are circled in black and numbered in (c) and indicated by black squares in (a). Group numbering corresponds to the order of sites in W in Fig. 3(a), from top to bottom, and group numbers are those to which Figs. 7 and 8 refer. In (b) and (c) the protonation configuration is that modeled to be the most common one at pH 7.1.
