Fig. 1. Serum HBsAg, HBcrAg and HBeAg reduction in human patients treated with a single dose of ARC-520.
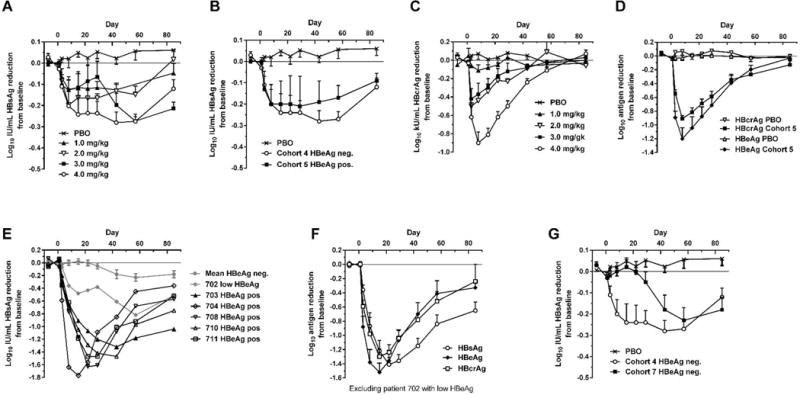
CHB patients were given a single intravenous dose of 1 to 4 mg/kg ARC-520 on a background of daily oral NUCs. HBsAg (A) or HBcrAg (C) reduction in CHB patients that were HBeAg negative, NUC-experienced and received single doses of 1 to 4 mg/kg (cohorts 1 to 4). (B) HBsAg reduction in CHB patients that were HBeAg negative and NUC-experienced (cohort 4) or HBeAg positive and NUC-experienced (cohort 5) that received a single dose of 4 mg/kg. (D) HBcrAg and HBeAg reduction in CHB patients that were HBeAg positive, NUC-experienced and received a single 4 mg/kg dose (cohort 5). (E) HBsAg reduction for individual CHB patients that were HBeAg positive in cohort 7. (F) HBsAg, HBeAg and HBcrAg reductions in CHB patients that were HBeAg positive, treatment naïve, and received a single 4 mg/kg dose (cohort 7). (G) HBsAg reduction in CHB patients that were HBeAg negative and treatment naïve (cohort 7) or NUC experienced (cohort 4). PBO, patients on NUC therapy given placebo injection; HBcrAg, HBV core-related antigen. Error bars show SEM.
