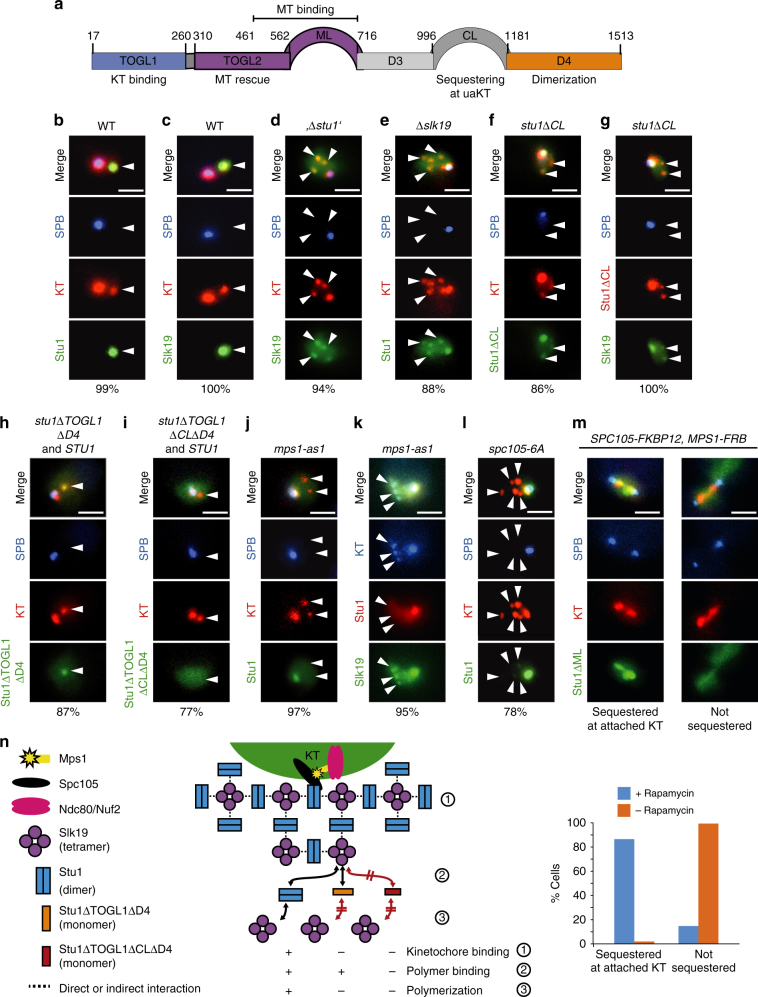
Fig. 1.
Dependencies of Stu1 and Slk19 localization and sequestering to uaKTs. a Domain structure and function of Stu18. b–m Genotypes of the strains revealing the fluorescent fusion proteins are listed in Supplementary Table 1. The percentage of cells revealing the depicted phenotype is indicated. Statistics see Supplementary Table 3. Bars, 2 µm. b–l Cells with the indicated genetic background were analyzed 3–4 h after the release from G1 into nocodazole. White arrowheads indicate uaKTs. b Stu1 is sequestered at uaKTs6. c Slk19 accumulates at uaKTs. d The accumulation of Slk19 at uaKTs depends on Stu1., ∆stu1’ indicates that Stu1 was depleted. e The sequestering of Stu1 at uaKTs depends on Slk19. f The CL domain of Stu1 is required for Stu1 sequestering8. g The CL domain of Stu1 is required for the accumulation of Slk19. h Stu1∆TOGL1∆D4 can localize to uaKTs in the presence of WT Stu1. i Stu1∆TOGL1∆CL∆D4 cannot localize to uaKTs even in the presence of WT Stu1. j The localization of Stu1 depends on Mps1. 1NM-PP1 was added after the G1 release. k Sequestering of Slk19 but not the basal localization of Slk19 depends on Mps1. Cells were treated as in j. l Mutating the six MELT Mps1 phosphorylation sites in Spc10519 to alanine (spc105-6A) prevents Stu1 sequestering. WT Spc105 in the background was depleted during the G1 arrest and Cdc20 was depleted with the G1 release to prevent the SAC-deficient cells from progressing into anaphase. m Stu1∆ML is sequestered at attached KTs when Mps1 is localized to Spc105 ectopically. Cells were arrested in metaphase by Cdc20 depletion and rapamycin was added as indicated. WT Stu1 was present in the background. n Model depicting the sequestering of Stu1 at uaKTs. In the absence of KT-MT interaction, Ndc80c-localized Mps1 phosphorylates Spc105 and thus prompts Stu1 localization to uaKTs. A putative conformational change that involves the CL domain triggers the interaction of Stu1 and Slk19. Propagated conformational changes in Slk19 and Stu1 lead to co-polymerization. Stu1∆TOGL1∆D4, which is unable to bind to uaKTs alone8, can bind to the Slk19 endpoints of the polymer, whereas Stu1∆TOGL1∆D4∆CL cannot (see h and i)