Figure 6.
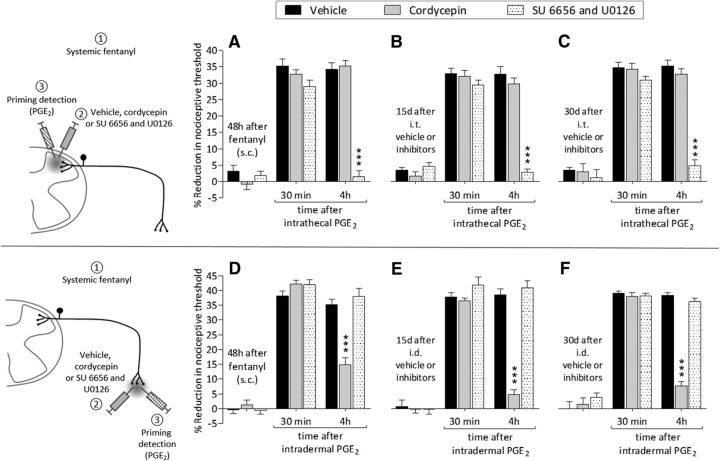
Systemic fentanyl induces priming at central and peripheral terminals. A, Rats were treated with subcutaneous fentanyl (20 μg/kg, × 4, every 15 min). Forty-eight hours later, when the mechanical nociceptive threshold were not different from pre-fentanyl baseline (before fentanyl: 142.8 ± 1.9 g; 48 h after fentanyl: 143.3 ± 1.7 g), vehicle (black bars; 10 μl), cordycepin (gray bars; 4 μg/10 μl) or the combination (dotted bars) of SU 6656 (10 μg/5 μl) and U0126 (10 μg/5 μl) were injected intrathecally followed by PGE2 (400 ng/10 μl) injected at the same site. Mechanical nociceptive threshold was evaluated 30 min and 4 h after intrathecal PGE2. Two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, showed that, whereas the hyperalgesia induced by PGE2 in the groups treated with vehicle or cordycepin was still present at the fourth hour, in the group pretreated with the combination of SU 6656 and U0126, it was completely blocked (F(2,30) = 304.62, ***p < 0.0001, when the hyperalgesia in the vehicle- and the combination of SU 6656 and U0126-treated groups is compared at the fourth hour after intradermal PGE2). B, Fifteen days after intrathecal treatment with vehicle, cordycepin, or the combination of SU 6656 and U0126, when the mechanical nociceptive threshold was not different from the pre-vehicle/inhibitors baseline (t(5) = 1.815; p = 0.1291, for the vehicle-treated group, t(5) = 0.8771; p = 0.4206, for the cordycepin-treated group, and t(5) = 0.8647; p = 0.4267, for the combination of SU 6656 and U0126-treated group, when the mechanical nociceptive threshold is compared before and after treatments; paired Student's t test), PGE2 (400 ng/20 μl) was again injected intrathecally. In the group previously treated with the combination of SU 6656 and U0126, PGE2-induced hyperalgesia was not present at the fourth hour (F(2,30) = 311.24, ***p < 0.0001, when the hyperalgesia in the vehicle-treated and the combination of SU 6656 and U0126-treated groups is compared at the fourth hour after intrathecal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). C, Thirty days after intrathecal treatment with vehicle, cordycepin, or the combination of SU 6656 and U0126, PGE2 (400 ng) was again injected intrathecally. In the group previously treated with the combination of inhibitors, the prolongation of PGE2-induced hyperalgesia was markedly inhibited at the fourth hour (F(2,30) = 194.44, ***p < 0.0001, when the hyperalgesia in the vehicle- and the combination of inhibitors-treated groups is compared at the fourth hour after intrathecal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), whereas in the groups treated with vehicle or the combination of SU 6656 and U0126 the hyperalgesia was present at the fourth hour. These data support the suggestion that systemic fentanyl produces type II priming in the central terminal. D, A different group of rats were also treated with subcutaneous fentanyl (20 μg/kg, × 4, every 15 min). Forty-eight hours later, when mechanical nociceptive threshold was not different from pre-fentanyl baseline (before fentanyl: 137.9 ± 1.5 g; 48 h after fentanyl: 135.7 ± 2.1 g), vehicle (black bars; 5 μl), cordycepin (gray bars; 1 μg/5 μl), or the combination (dotted bars) of SU 6656 (1 μg/3 μl) and U0126 (1 μg/3 μl) were injected intradermally followed 10 min later by PGE2 (100 ng/5 μl) injected at the same site. Mechanical nociceptive threshold was evaluated 30 min and 4 h after intradermal PGE2. In the group treated with intradermal cordycepin, the prolongation of PGE2-induced hyperalgesia was markedly attenuated (F(2,30) = 459.43, ***p < 0.0001, when the hyperalgesia in the vehicle- and the cordycepin-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), whereas in the groups treated with vehicle or the combination of SU 6656 and U0126, the hyperalgesia was present at the fourth hour. E, Fifteen days after intradermal treatment with vehicle, cordycepin, or the combination of SU 6656 and U0126, when the mechanical nociceptive threshold was not different from the pre-vehicle/inhibitors baseline (t(5) = 0.7133; p = 0.5076, for the vehicle-treated group, t(5) = 0.3561; p = 0.7363, for the cordycepin-treated group, and t(5) = 1.504; p = 0.1929, for the combination of SU 6656 and U0126-treated group, when the mechanical nociceptive threshold is compared before and after treatments; paired Student's t test), PGE2 (100 ng/5 μl) was again injected intradermally. The prolongation of PGE2-induced hyperalgesia was still inhibited in the group previously treated with cordycepin (F(2,30) = 389.49, ***p < 0.0001, when cordycepin-treated is compared with the vehicle-treated group at the fourth hour after the injection of PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), but not in the groups treated with vehicle or the combination of SU 6656 and U0126. F, Thirty days after intradermal vehicle, cordycepin, or the combination of SU 6656 and U0126, PGE2 (100 ng) was again injected intradermally. Prolongation of PGE2-induced hyperalgesia was markedly inhibited in the group previously treated with cordycepin (F(2,30) = 406.02, ***p < 0.0001, when the hyperalgesia in the cordycepin-treated group is compared with vehicle at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), whereas in the groups treated with vehicle or the combination of SU 6656 and U0126, the hyperalgesia was present at the fourth hour. These data indicate that systemic fentanyl produces type I priming in the peripheral terminal of the nociceptor (n = 6 paws/6 rats per group).