Figure 1.
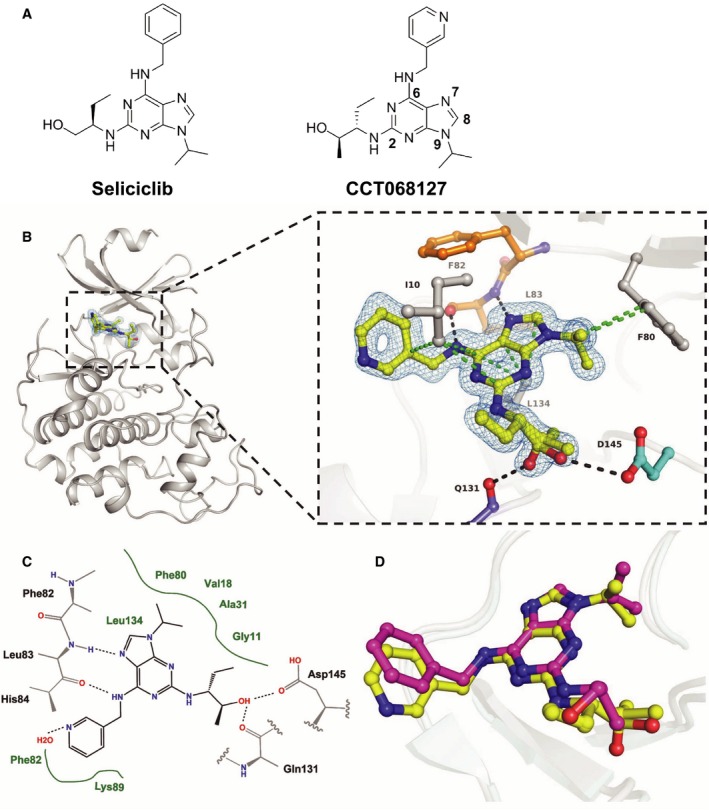
Enhanced potency of CCT068127 over seliciclib is achieved by additional ligand interactions with CDK2 and CDK9. (A) Chemical structures of seliciclib and CCT068127; numbering of the purine scaffold is indicated for CCT068127. (B) Secondary structure representation of human CDK2 in complex with CCT068127 determined by X‐ray crystallography at 1.3 Å resolution. The inset shows the binding interactions of CCT068127 within the ATP binding pocket. The hinge region is indicated in orange, Asp145 of the DFG motif in cyan, and CCT068127 in yellow. Displayed in blue is the 2Fo‐Fc electron density, contoured at 1σ around CCT068127. The data and refinement statistics are shown in the Supporting information (Table S3). The hydrogen bonding and van der Waals (hydrophobic) interactions are shown as black and green dotted lines, respectively. PDB: 5MHQ. (C) Schematic presentation of the binding interactions between CCT068127 and CDK2. (D) Alignment of CCT068127 (yellow) and seliciclib (magenta) (PDB: 3DDQ) bound to CDK2.
