Figure 2.
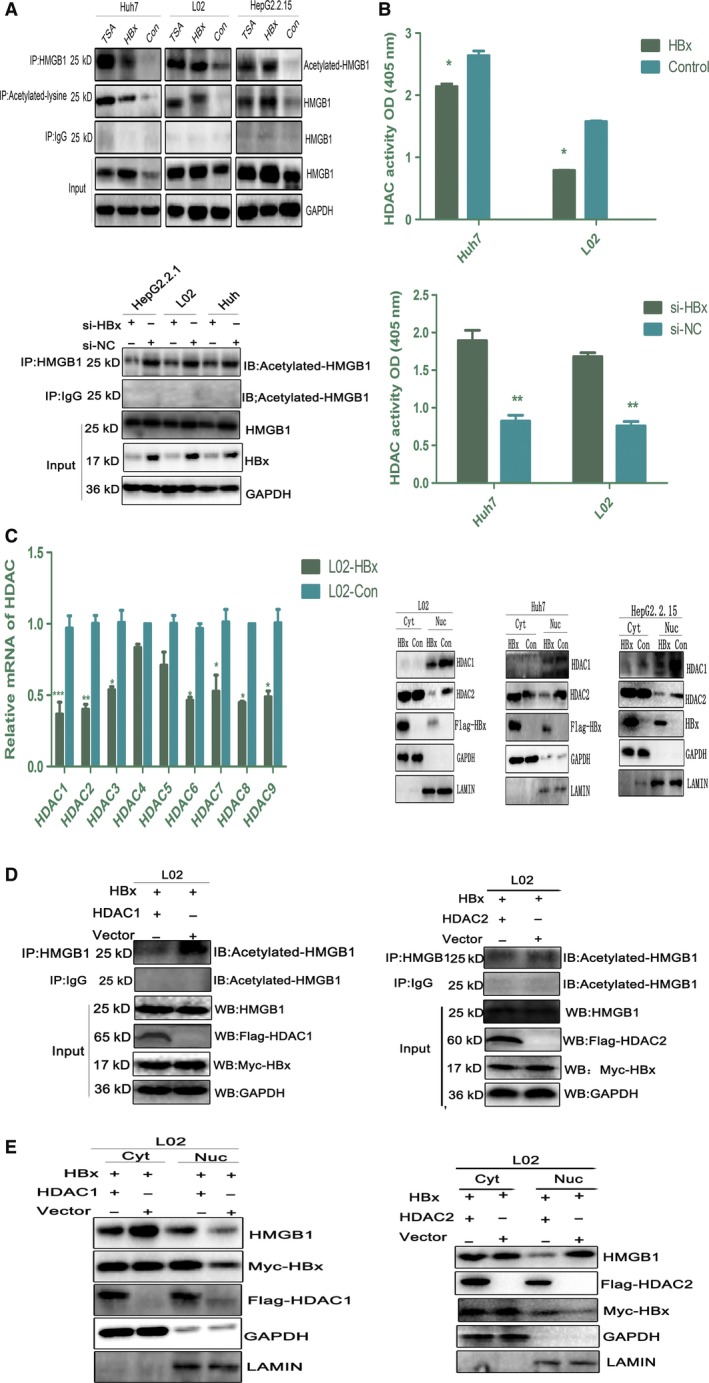
HBx induces HMGB1 acetylation by regulating HDAC activity and expression. (A) HBx promotes the acetylation of HMGB1. TSA (1 μm, 24 h) treatment was used as a positive control. Whole lysates of HBx‐L02, HBx‐Huh7, and HepG2.2.15 cells were immunoprecipitated with an acetylated lysine or HMGB1 antibody and then subjected to immunoblot analysis with the indicated antibodies. Samples were pulled down with anti‐HMGB1 and immunoblotted with anti‐acetylated lysine following HBx knockdown via siRNA treatment for 72 h. (B) HDAC activity was determined by colorimetric assay after HBx overexpression or knockdown. *P < 0.05, **P < 0.01, n = 3. (C) RT‐PCR analysis of HDAC1–9 expression in HBx‐L02 and Vector‐L02 cells. *P < 0.05, **P < 0.01, n = 3. Immunoblot analysis of nuclear/Cyt HDAC1 or HDAC2 expression in HBx‐L02 and Vector‐L02 cells. (D and E) L02 cells were cotransfected with Myc‐tagged HBx (1.5 μg) and Flag‐tagged HDAC1 (1.5 μg) or Flag‐tagged HDAC2 (1.5 μg) for 48 h. Whole lysates were immunoprecipitated with anti‐HMGB1 and immunoblotted with anti‐acetylated lysine. Nuclear/Cyt HMGB1 distribution was then analyzed by western blot.
