Figure 4.
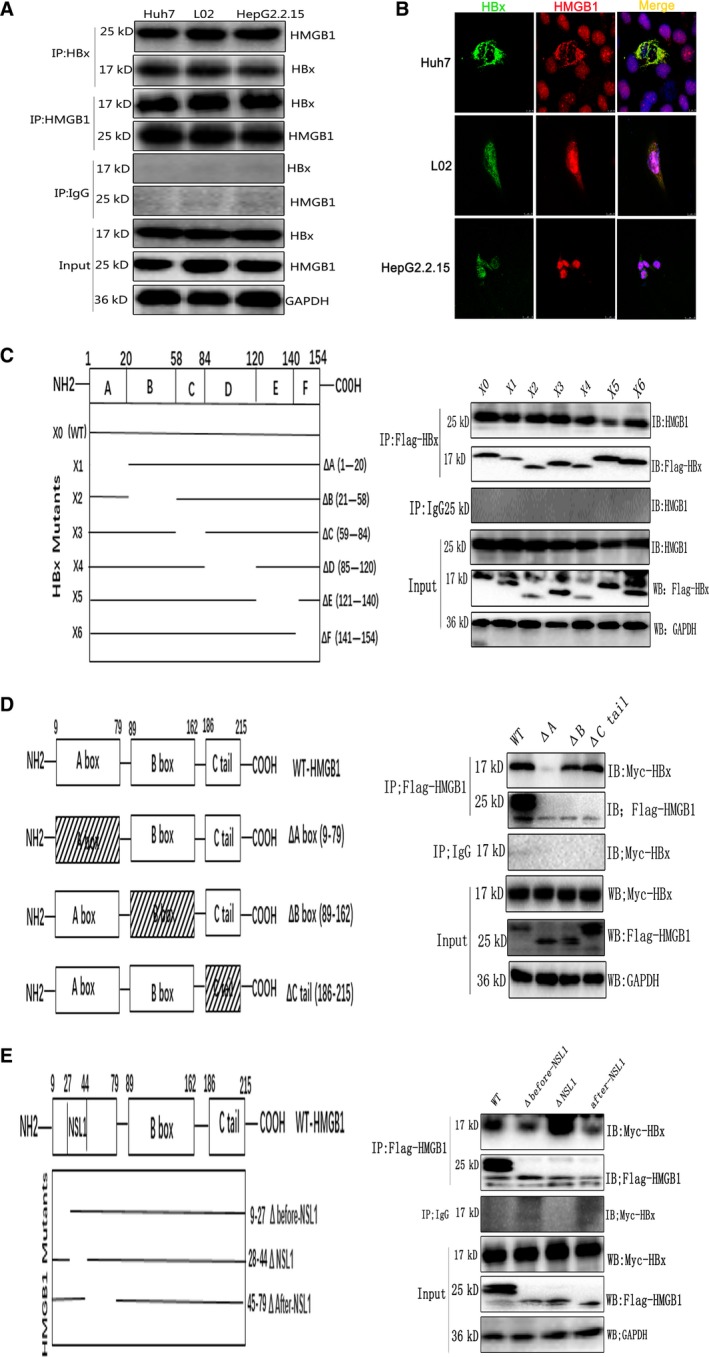
HBx binds HMGB1 in the cytoplasm. (A). Whole‐cell extracts of HBx‐Huh7, HBx‐L02, and HepG2.2.15 cells were immunoprecipitated with the indicated antibodies, and the immunoprecipitates were analyzed by immunoblotting. (B) Colocalization of HBx and HMGB1 was analyzed by confocal microscopy. (C) Scheme of HBx mutants. The 154‐amino‐acid WT HBx sequence (X0) was divided into six regions (A–F). The line indicates the portion of HBx present in the mutant, with the deleted region shown as a gap. 293T cells were transfected with Flag‐tagged full‐length HBx and deletion (Δ) mutants (X1–X6) of HBx. Cell lysates were immunoprecipitated with anti‐Flag and probed by western blot with anti‐HMGB1. (D) Scheme of HMGB1 mutants. The 215‐amino‐acid WT HMGB1 sequence was divided into three regions, including the A box, B box, and C‐tail. The box with hatch marks indicates the deleted region of HMGB1. 293T cells were transfected with Myc‐HBx and Flag‐tagged WT HMGB1 or the A box, B box, or C‐tail deletion of HMGB1. Cell lysates were immunoprecipitated with anti‐Flag and probed by western blot with anti‐Myc. (E) The 70‐amino‐acid A box in HMGB1 was further divided into three regions based on the location of NSL1, that is, one NSL1‐containing domain, one domain before NSL1 and one domain after NSL1. The line indicates the portion of HMGB1 present in the mutant, with the deleted region shown as a gap. 293T cells were transfected with Myc‐HBx and Flag‐tagged WT HMGB1 or the NSL1, before‐NSL1, or after‐NSL1 deletion of HMGB1. Cell lysates were immunoprecipitated with anti‐Flag and probed by western blot with anti‐Myc.
