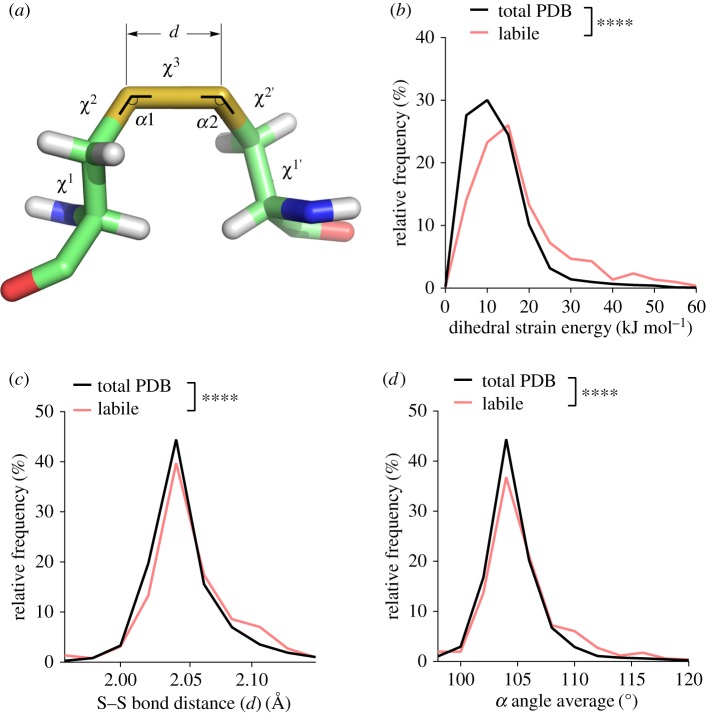
Figure 2.
The labile disulfides are characterized by high dihedral strain energy, elongation of the sulfur–sulfur bond distance and stretching of the neighbouring bond angles. (a) Angles and distances of the cystine residue. The values α1 and α2 represent the two relevant bending angles of the disulfide, and the five dihedral angles are χ1, χ2, χ3, χ2′ and χ1′. d is the sulfur–sulfur bond length. (b) Relative frequency of DSE ranging from 0 to 60 kJ mol−1. The DSE was significantly increased for labile disulfides compared to all disulfide bonds in the PDB (p < 0.0001). (c) The relative frequency of the sulfur–sulfur bond distance ranging from 1.96 to 2.14 Å is shown. An increase in sulfur–sulfur bond distance is observed for labile disulfide bonds (p < 0.0001). (d) The average of both α angles was calculated for each disulfide bond. Shown is the relative frequency of the average angle ranging from 95 to 120°. Angles are increased for labile disulfide bonds (p < 0.0001). T-tests were used to compare total PDB to labile disulfides.