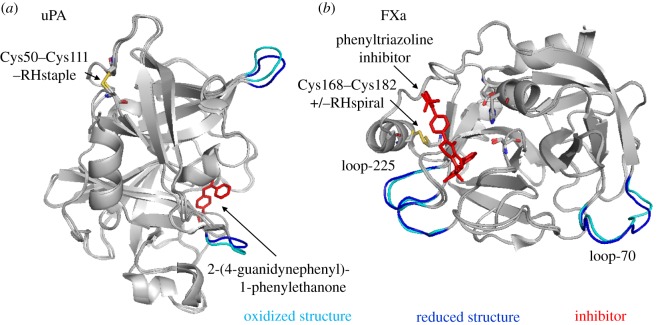
Figure 4.
Conformational changes in proteins of the coagulation and complement pathways upon cleavage of the labile disulfide bonds. Structures of oxidized (cyan) and reduced (blue) proteins are aligned. Labile disulfides and their configurations are shown. The PDB identifiers are indicated in table 1. (a) uPA is shown in complex with the inhibitor, 2-(4-guanidynephenyl)-1-phenylethanone. The redox state of the Cys50–Cys111 disulfide influences the positions of loops surrounding the active site. (b) Factor Xa is shown in the presence of a phenyltriazoline inhibitor. The catalytic triad consisting of His57, Asp102 and Ser195 is shown in stick presentation. The redox state of Cys168–Cys182 influences slight conformational changes in Ca2+-binding loop-70 and Na2+-binding loop-225.