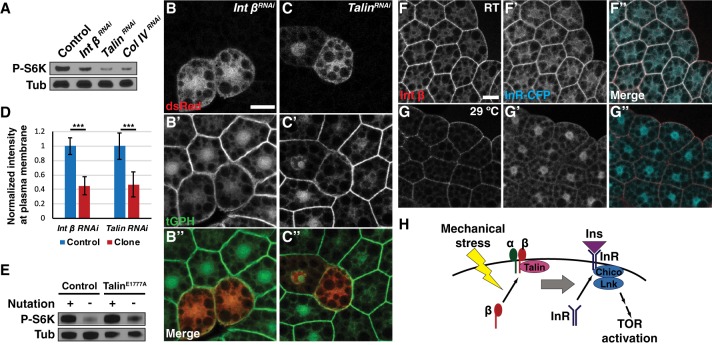
Figure 5.
Integrin components are necessary and sufficient to activate insulin signaling by regulating InR localization. (A) Ubiquitous depletion of Integrin β, Talin, or collagen IV using hs-Gal4 decreased fb-TOR activity. (B–D) Depletion of Integrin β (B) or Talin (C) in dsRed-marked clones of the fat body led to significantly decreased localization of GFP-PH at the plasma membrane. Bar, 20 µm. The graph in D shows normalized membrane signal intensity of GFP-PH in response to depletion of Integrin β (n = 50) or Talin (n = 30). (***) P < 0.001, Student's t-test. (E) Ubi::TalinE1777A partially rescued fb-TOR activity in the absence of nutation. (F,G) Membrane localization of InR-CFP was reduced in temperature-sensitive integrin β mutants (mysts1) when incubated ex vivo at the restrictive temperature (29°C) (G) compared with the permissive temperature (room temperature [RT]) (F). Bar, 20 µm. (H) Model for this study. Mechanical stress is sensed and transmitted downstream by components of integrin signaling, including Integrin β and Talin. Activation of integrin signaling leads to localization of InR at the plasma membrane, thereby promoting the access of InR to extracellular insulin and leading to activation of canonical insulin signaling. Integrin signaling may promote the delivery or inhibit the retrieval of InR to or from the cell membrane. Mechanical stress also acts on insulin signaling at a step further downstream, between PI3K and Rheb.