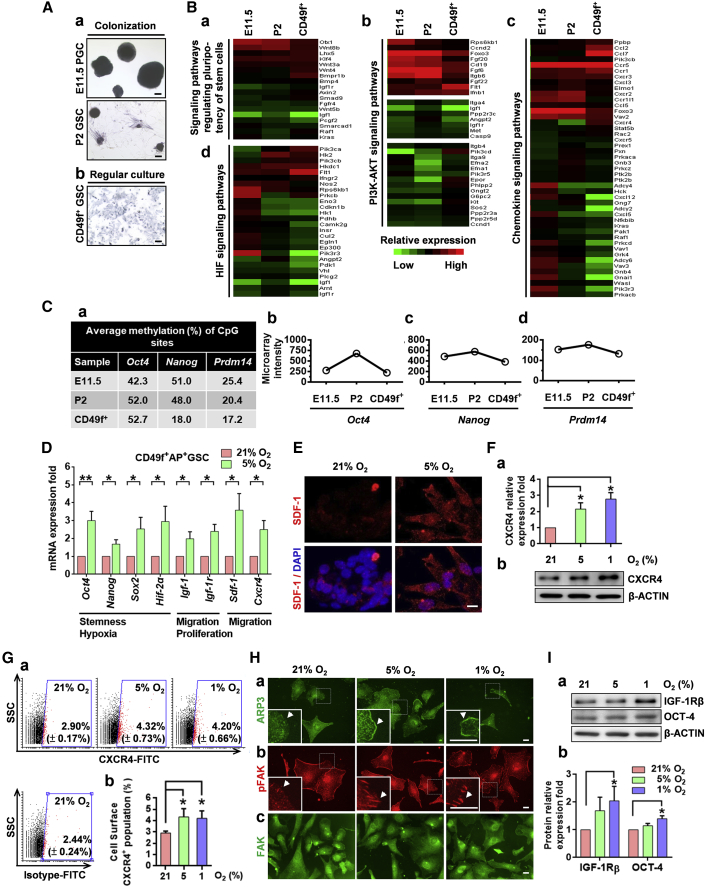
Figure 3.
Hypoxia Increased the Expression Levels of Stemness/Migration-Related Proteins in PGC-Like CD49f+AP+GSCs
(A) AP staining (in blue). (a) Colony formation of E11.5 PGCs and P2 GSCs under 5% O2 hypoxia. (b) Regular culture of purified CD49f+GSCs. Scale bars, 100 μm.
(B) Heatmap of selected gene expression patterns in E11.5 PGCs, P2 GSCs, and purified CD49f+GSCs. Signaling pathways of GO for (a) regulating the pluripotency of stem cells, (b) PI3K-AKT, (c) chemokine, and (d) HIF.
(C) (a) Bisulfite genomic sequencing. (b–d) Gene expression of Oct4, Nanog, and Prdm14 in E11.5 PGCs, P2 GSCs, and purified CD49f+GSCs.
(D) The mRNA levels of stemness-, hypoxia-, proliferation-, and migration-related genes of CD49f+AP+GSCs under different oxygen concentrations (qRT-PCR analysis). Student's t test.
(E) Immunofluorescence staining of SDF-1 in CD49f+AP+GSCs under 21% and 5% O2. Scale bar, 10 μm.
(F) Protein levels of CXCR4 under different oxygen concentrations were analyzed through western blotting. The quantification analysis result is shown. One-way ANOVA.
(G) (a) Cell surface levels of CXCR4 of CD49f+AP+GSCs under different oxygen concentrations were analyzed through flow cytometry. The percentage of CXCR4-positive cells (red dot) is shown. (b) Quantitative analysis of (a). One-way ANOVA.
(H) Cellular localization of the migration-related markers (a) ARP3, (b) pFAK, and (c) FAK under different oxygen concentrations. Arrowheads indicate the specific cellular protein localization of ARP3 and pFAK. Scale bars, 20 μm.
(I) (a) Protein levels of IGF-1Rβ and OCT4 under different oxygen concentrations were analyzed using western blotting. (b) Quantification analysis results. One-way ANOVA.
For all quantifications, data are the means ± SEM of at least three independent experiments. ∗p < 0.05, ∗∗p < 0.01. See also Figures S2–S6 and Tables S3 and S4.