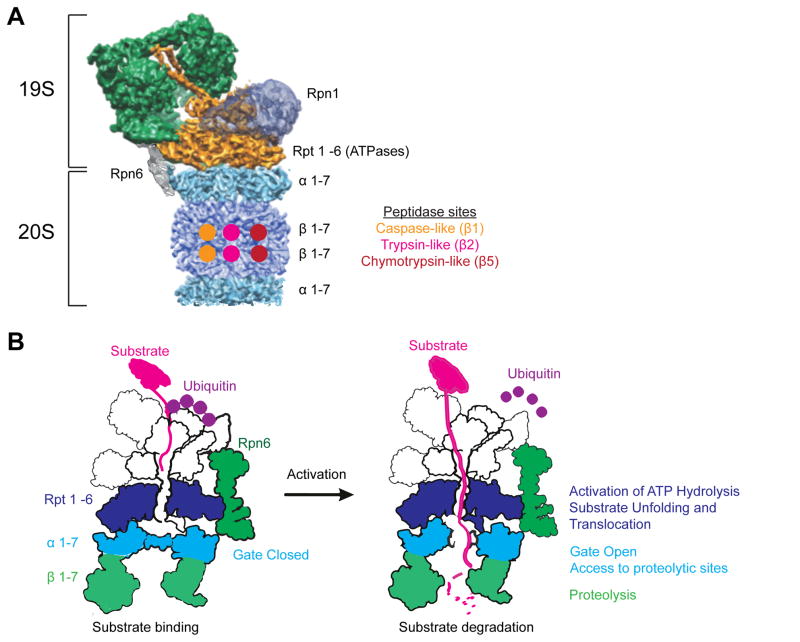
Figure 1. Structures of the 26S proteasome.
A.) The 26S proteasome is composed of the 20S core particle and a 19S regulatory particle attached to one or both ends. The 20S has seven α subunits (light blue), whose N-termini form a gate that prevents nonspecific protein degradation, and seven β subunits (dark blue), three of which contain proteolytic active sites with distinct substrate preference (indicated). The 19S has six ATPase subunits (Rpt 1-6) (orange), which unfold and translocate substrates in an ATP-dependent manner into the 20S for proteolysis. Three of these ATPase subunits (Rpt 3, 5, and 6) are reported to be phosphorylated by kinases discussed here. Rpn6 is phosphorylated by PKA and is unusual because it interacts with subunits of both the 19S ATPase ring and 20S α ring.
B.) A ubiquitinated substrate is bound by one of the ubiquitin receptor subunits. If the substrate has an unstructured region, it becomes tightly bound by the proteasome, committed to degradation, and the 19S ATPase subunits (Rpt 1 - 6) are activated. Degradation does not occur every time a ubiquitinated substrate binds the proteasome because deubiqutination can occur prior to the commitment step, resulting in substrate release. If committed to degradation, the substrate is translocated in an ATP-dependent manner into the 20S where it is hydrolyzed to small peptides by the three proteolytic sites. It is currently unknown how phosphorylation of a subunit either speeds up or slows down this degradation process.