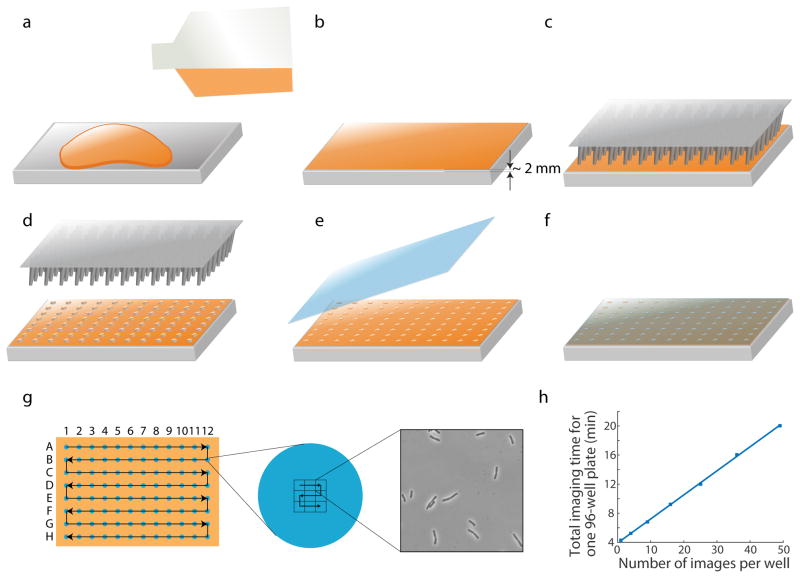
Figure 1. Schematic of pad preparation and imaging.
(a) Melted agar is poured onto the bottom surface of a Singer PlusPlate. (b) Melted agar is spread evenly by gently tilting and shaking the plate. A flat agar surface is generally achieved when the plate is left on the benchtop and solidifies without disturbance. The agar layer is ~2 mm in thickness. (c, d) Bacteria cultures are transferred onto an agar pad using a 96-well replicator pin. (e, f) After the agar pad absorbs all the liquid (~5–10 min depending on temperature), a large glass cover slip is used to cover the agar surface. (g) Schematic of stage movement during image acquisition for a 96-well plate. Left: to minimize travel distance, the stage moves across the wells in a zigzag manner, first from A1 to A12, then backward from B12 to B1, etc. Middle: for each strain, the stage moves across an imaging grid in a similar zigzag pattern. An example of a 3x3 grid is shown. The grid size is enlarged relative to the droplet size for illustration purposes. Right: a typical image acquired by SLIP. (h) For a 96-well plate, total image acquisition time scales linearly with number of images per strain, with a slope of 200 ms/strain/image or 0.33 min for each additional image across 96 strains.