This study assesses whether the MGMT protein expression in patients with late-stage gastric cancer is associated with prognosis or the need for fluorouracil-based adjuvant chemotherapy.
Key Points
Questions
What role does the O6-methylguanine-DNA methyltransferase (MGMT) protein expression play in gastric cancer, and do MGMT-positive and MGMT-negative tumors respond differently to fluorouracil-based adjuvant chemotherapy?
Findings
In this study involving 445 postoperative patients with resectable gastric cancer, positive expression of MGMT was an independent, favorable prognostic factor and identified a subgroup of patients with stage II disease who could benefit more from fluorouracil-based adjuvant chemotherapy.
Meaning
In resectable gastric cancer, detection of MGMT may be of use to prognosis prediction, and fluorouracil-based adjuvant chemotherapy is especially recommended for patients with stage II, MGMT-positive gastric cancer.
Abstract
Importance
Loss of O6-methylguanine-DNA methyltransferase (MGMT) protein expression has been reported in several malignant tumors and predicts dismal survival outcomes. In gastric cancer, existing studies on this topic are limited and the association between MGMT and fluorouracil-based adjuvant chemotherapy remains obscure.
Objective
To investigate the postoperative prognostic significance of MGMT in patients with resectable gastric cancer and its responsiveness to fluorouracil-based adjuvant chemotherapy.
Design, Setting, and Participants
This study recruited 496 patients with resectable gastric cancer who underwent radical gastrectomy between August 1, 2007, and December 30, 2008, at Zhongshan Hospital at Fudan University in Shanghai, China. However, 468 of 496 patients had comprehensive information about chemotherapy, clinicopathological data, and survival outcomes for complete analysis. In this study, we used tissue microarrays (Shanghai Outdo Biotech Co, Ltd), and 15 dots of the 468 patients were lost after immunohistochemistry. Additionally, 8 patients of TMN stage IV were excluded. Consequently, 445 patients were included in this study. Patients were randomly divided into a discovery data set (n = 200) and a validation data set (n = 245), and the range of follow-up time was from 2 to 76 months for the discovery group and 2 to 79 months for the validation group. The immunoreactivity for MGMT in cancer cells was reviewed under a light microscope by 2 pathologists who were blinded to the clinicopathological data. The association of MGMT expression with clinicopathological characteristics and measures and prognosis was inspected. Data and specimens were collected from patients from the date of surgery to April 25, 2014. Data analysis took place from May 9, 2016, to July 15, 2016.
Main Outcomes and Measures
Estimates of overall survival on the basis of MGMT expression and hazard ratio (HR) for estimates of overall mortality risk.
Results
Of the 445 patients included in the study, 315 (70.8%) were men, and the mean (SD) age of all patients was 60 (12) years. Positive expression of MGMT indicated better overall survival for patients with stage II or III gastric cancer in both the discovery data set (HR, 0.52; 95% CI, 0.32-0.84; P = .003) and the validation data set (HR, 0.63; 95% CI, 0.43-0.93; P = .01). Multivariate analysis identified MGMT expression and TNM stage as 2 independent prognostic factors for overall survival. In stage II disease, the benefit from fluorouracil-based adjuvant chemotherapy was superior among MGMT-positive patients (HR, 0.35; 95% CI, 0.13-0.95; P = .007 for interaction) compared with MGMT-negative patients.
Conclusions and Relevance
Positive expression of MGMT in gastric cancer was identified as an independent, favorable prognostic factor. Incorporating MGMT expression into the current TNM staging system could lead to better prognostic accuracy. These findings should be confirmed within the framework of randomized clinical trials associated with genomic DNA sequencing studies.
Introduction
Most of the developed countries in North America and northern Europe have seen a steady decline in gastric cancer incidence and mortality rates in the past 60 years.1 In a less developed country such as China, however, gastric cancer still ranks as the second most common cancer diagnosed and is a leading cause of cancer death.2 As the only feasible curative treatment, surgical resection is generally advised for patients with gastric cancer.3 Unfortunately, the rate of relapse is high among patients with advanced gastric cancer, to whom adjuvant chemotherapy based on fluorouracil is commonly given as a first-line postoperative treatment.4 As one of the most commonly used antineoplastic agents, fluorouracil has been applied for the treatment of various cancers in the past 40 years and remains the standard first-line choice for adjuvant chemotherapy.5 However, the role of adjuvant therapy in gastric cancer is controversial because many randomized clinical trials have found it lacking survival benefit.6 Thus, an urgent need exists for further classification of gastric cancer, one that can accurately be correlated with patient outcomes and treatment response.
The O6-methylguanine-DNA methyltransferase (MGMT), also known as O6-alkylguanine-DNA alkyltransferase,7 is a protein that in humans is encoded by the MGMT gene (OMIM NM_002412.4) and functions as a DNA repairer capable of removing methyl groups and larger adducts at the O6 position of guanine.8 Through a reaction where each lesion is repaired at the cost of 1 MGMT molecule being inactivated, MGMT can transfer the alkyl group from the O6 position of guanine in DNA to an active cysteine within its own sequence.8,9 Because the mutation or deletion of the MGMT gene is not common, lack of MGMT may consequently result from changes that do not alter the genetic information within the cell.7 In humans, DNA methylation is the primary type of such epigenetic modifications and ultimately can lead to tumorigenesis.10 Methylation of generally unmethylated sites, known as cytidine phosphate guanosine islands, in the promoter regions of tumor-suppressor and DNA-repair genes has proven to be correlated with the loss of expression of these genes within cancer cell lines and primary tumors.10 In the MGMT gene, methylation of the cytidine phosphate guanosine island can prevent gene transcription, and in cell lines that are unable to repair alkylation of O6-methylguanine, the promoter of MGMT is always observed methylated.8,11 Methylation of the MGMT gene has been reported in several malignant tumors. In gliomas and colorectal cancers, MGMT gene methylation was found in 38% of the tumor, whereas in non–small cell lung carcinomas, head and neck carcinomas, and lymphomas, MGMT gene methylation was confirmed in 23% to 28% of tumors.8,12 However, the association of MGMT expression with gastric cancer was still obscure, and precise mechanisms underlying MGMT in gastric cancer progression required further investigation.
We conducted a study to determine whether MGMT expression could be used as an independent prognosticator for patients with resectable gastric cancer and whether MGMT could identify the patients who might benefit more from typical postoperative fluorouracil-based adjuvant chemotherapy. We previously published related studies from the same overall patient sample that were primarily focused on other biomarkers, the tumor microenvironment, and immune signatures in gastric cancer.13,14,15,16,17,18,19,20,21 In this study, we assessed the clinical importance of the tumor mutation burden associated with MGMT protein in gastric cancer and provide a possible predictive system to evaluate outcomes for patients with gastric cancer.
Methods
Patients and Tissue Samples
This study recruited a sample of 496 patients with resectable gastric cancer who underwent surgery at Zhongshan Hospital at Fudan University in Shanghai, China. However, 468 of 496 patients had comprehensive information about chemotherapy, clinicopathological data, and survival outcomes for complete analysis. For these 468 patients, we included assessment of tissue microarrays that were initially constructed by the Department of Pathology at the Zhongshan Hospital. However, the quality of these tissue microarrays was inadequate for analysis. Thus, we sent tissue specimens for the 468 patients to Shanghai Outdo Biotech Co, Ltd, which constructed new tissue microarrays. Two pathologists at this company evaluated the immunohistochemistry scores, and then 2 coauthors confirmed these scores, as described further below. We paid for this commercial service. After immunohistochemistry had been assessed, data for the specimens for 15 patients were lost, and we excluded an additional 8 patients with TNM stage IV gastric cancer. Consequently, we included a cohort of 445 patients with resectable gastric cancer who underwent radical gastrectomy with standard D2 lymphadenectomy between August 1, 2007, and December 30, 2008. The Clinical Research Ethics Committee of Zhongshan Hospital approved this study, and patients provided written informed consent before surgery for the use of their resected samples. Data and specimens were collected from patients from the date of surgery to April 25, 2014. Data analysis took place from May 9, 2016, to July 15, 2016.
Clinicopathological characteristics and measures, including age, sex, tumor localization, tumor size, differentiation, Lauren classification,22 and TNM stage, were retrospectively collected from each patient enrolled in the study. The specimens were evaluated independently by 2 of us who were gastroenterology pathologists (H. Liu and H. Li) blind to patients’ clinicopathological data, and the TNM staging system was evaluated according to the 2010 American Joint Committee on Cancer (AJCC) and International Union Against Cancer TNM staging system.23 The cohort was randomly divided into 2 independent data sets: the discovery data set (n = 200) and the validation data set (n = 245). Using the AJCC and International Union Against Cancer TNM staging system for gastric cancer, we categorized cases in the discovery data set as follows: 52 cases as stage I, 49 as stage II, and 99 as stage III. Similarly, we classified cases in the validation data set as follows: 60 cases as stage I, 55 as stage II, and 130 as stage III. After surgery, patients with advanced disease (stage II or III) were primarily given routine fluorouracil-based adjuvant chemotherapy. No radiotherapy was administered to any enrolled patients. The end point of interest was overall survival (OS), which was calculated from the date of gastrectomy to the date of death or to the date of the last follow-up.
Patients were observed from date of surgery until April 25, 2014. The median (range) follow-up time was 49 (2 -76) months for the discovery data set and 43 (2 -79) months for the validation data set. Gastric cancer tissues were collected from the gastrectomy specimens of the 445 patients, and noncancerous gastric tissues were obtained from the gastrectomy specimens of adjacent gastric cancer margins within 5 cm. All tissues were formalin-fixed and paraffin-embedded samples.
Tissue Microarray and Immunohistochemistry
Before the construction of tissue microarrays, 4-μm-thick sections were sliced from each tissue block and stained with hematoxylin-eosin for diagnostic confirmation and selection of the representative area for each tumor tissue. Then, tissue microarray blocks containing both tumor tissues and noncancerous gastric tissues were prepared by means of the method described previously24 (eMethods 1 in the Supplement).
The entire set of tissue specimens was processed and immunostained at the same time to guarantee an objective comparison between samples. Immunohistochemistry was performed at Shanghai Outdo Biotech Co, Ltd, as described elsewhere in this article24,25 (eMethods 2 in the Supplement).
Evaluation of Immunostaining Intensity
The MGMT protein was immunohistochemically stained yellowish to brown in the nuclei of normal cells and cancer cells. The immunoreactivity for MGMT in cancer cells was reviewed under a light microscope by 2 pathologists from Shanghai Outdo Biotech Co, Ltd. In addition, 2 of us (H. Liu and H. Li), who were blinded to the clinicopathological data, had confirmed all these immunohistochemistry scores on the basis of 2 pathologists’ work. The microarrays were scored independently and paired at the end. If the 2 scores for a certain sample were discordant, the final score was generated on the consensus reached by the 2 pathologists. All tumors in which the malignant epithelial component showed widespread nuclear expression of MGMT, either in all or most cancer cells, were scored as MGMT-positive. Conversely, all tumors where the malignant epithelial component either completely lacked MGMT expression or showed faint nuclear expression in only a few carcinoma epithelial cells were considered MGMT-negative. The correlation between MGMT expression and survival outcomes was tested by a third investigator (C.L.) who did not participate in the scoring process.
Statistical Analysis
Statistical analysis was performed with use of SPSS, version 21.0 (IBM). The Pearson χ2 test was applied for categorical variables, and continuous variables were analyzed through an unpaired, 2-tailed t test. Survival outcomes of patient subgroups were compared by means of Kaplan-Meier curves, log-rank tests, and multivariate analysis based on the Cox proportional hazards method. Interactions between the marker status and treatment were detected by means of the Cox model as well. Time-dependent area under the receiver operating characteristic curve (AUC) was computed with the use of the R software, version 3.3.2, due to a limitation in the earlier version of the R software that might not run the “survival” and “timeROC” package (R Foundation for Statistical Computing) successfully. Prognostic nomogram and calibration plots were established by the “rms” package (R Foundation for Statistical Computing). All statistical analyses were 2-sided, and 2-sided P < .05 was regarded as statistically significant.
Results
Association of MGMT Expression With Clinicopathological Characteristics
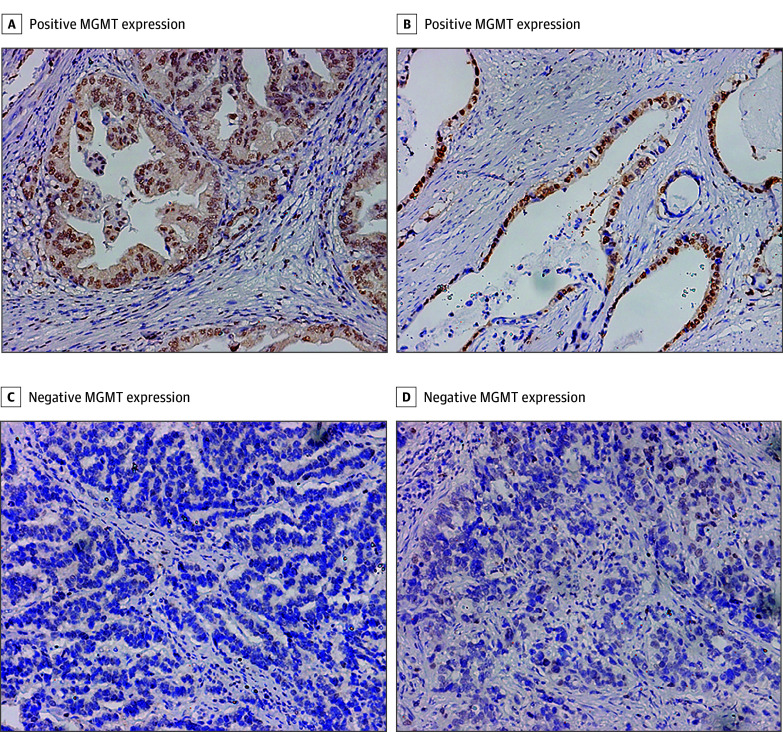
This study was carried out as described in Figure 1. The expression of MGMT was detected in 445 patients with resectable gastric cancer. Of the 445 patients included in the study, 315 (70.8%) were men, and the mean (SD) age of all patients was 60 (12) years. A total of 154 patients (34.6%) were MGMT-negative and 291 patients (65.4%) were MGMT-positive. The cohort was then randomly divided into 2 independent sets—a discovery data set (n = 200) and a validation data set (n = 245). A total of 65 patients (32.5%) in the discovery data set and 89 patients (36.3%) in the validation data set were MGMT-negative. The immunostaining for MGMT is shown in Figure 2. The comprehensive characteristics of patients, together with clinicopathological measures, are listed in eTable 1 in the Supplement.
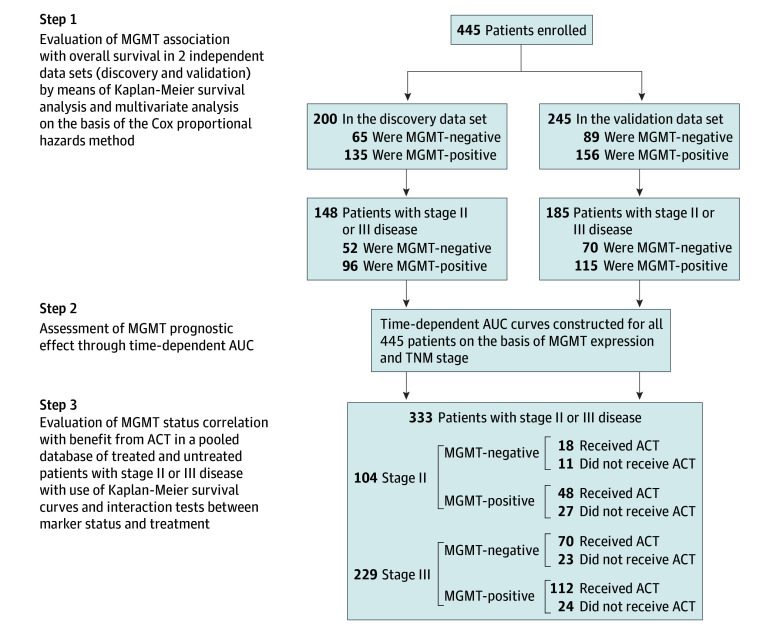
Figure 1. Study Design.
A cohort of 445 consecutive patients with resectable gastric cancer who underwent radical gastrectomy was enrolled in this study. The association between O6-methylguanine-DNA methyltransferase (MGMT) expression and overall survival was investigated in 2 independent, randomly assigned patient data sets: the discovery data set (n = 200) and the validation data set (n = 245). The association between MGMT protein expression and benefit from fluorouracil-based adjuvant chemotherapy was tested in a pooled database of 104 patients with TNM stage II disease and 229 patients with stage III disease from the 2 data sets. ACT indicates adjuvant chemotherapy; AUC, area under the receiver operating characteristic curve.
Figure 2. O6-Methylguanine-DNA Methyltransferase (MGMT) Expression in Gastric Cancer Tissues.
All specimens were stained using the immunohistochemistry techniques described in eMethods 2 of the Supplement and had an original magnification of ×200.
Kaplan-Meier Survival Curves and Log-Rank Test Results
To discover the prognostic capability of MGMT expression in patients with resectable gastric cancer, we applied Kaplan-Meier survival analysis to compare OS according to MGMT expression. In both the discovery data set (hazard ratio [HR], 0.49; 95% CI, 0.30-0.79; P < .001) and the validation data set (HR, 0.58; 95% CI, 0.40-0.84; P = .002), patients with MGMT-positive expression had obviously better OS (Figure 3) than those with MGMT-negative expression, indicating the potential clinical significance of MGMT for survival outcomes among patients with resectable gastric cancer. We further classified patients into an early-stage (TNM stage I) disease subgroup or an advanced-stage (TNM stage II or III) disease subgroup. Our analysis, which was validated, showed that OS was significantly better among the 96 patients in the discovery data set who were stage II or III with MGMT-positive tumors (HR, 0.52; 95% CI, 0.32-0.84; P = .003; Figure 3A). In the validation data set, we also observed such a phenomenon among the 115 patients with MGMT-positive tumors (HR, 0.63; 95% CI, 0.43-0.93; P = .01; Figure 3B). However, in stage I disease, neither the discovery data set nor the validation data set showed a significant difference in OS between MGMT-positive and MGMT-negative patients (HR, 0.64; 95% CI, 0.10-4.15; P = .61, and HR, 0.42; 95% CI, 0.11-1.63; P = .16; Figure 3). Consequently, MGMT-positive expression might act as a favorable prognostic factor for TNM stage II or III resectable gastric cancer.
Figure 3. Kaplan-Meier Survival Curves and Log-Rank Test to Evaluate the Prognostic Value of O6-Methylguanine-DNA Methyltransferase (MGMT) Status.
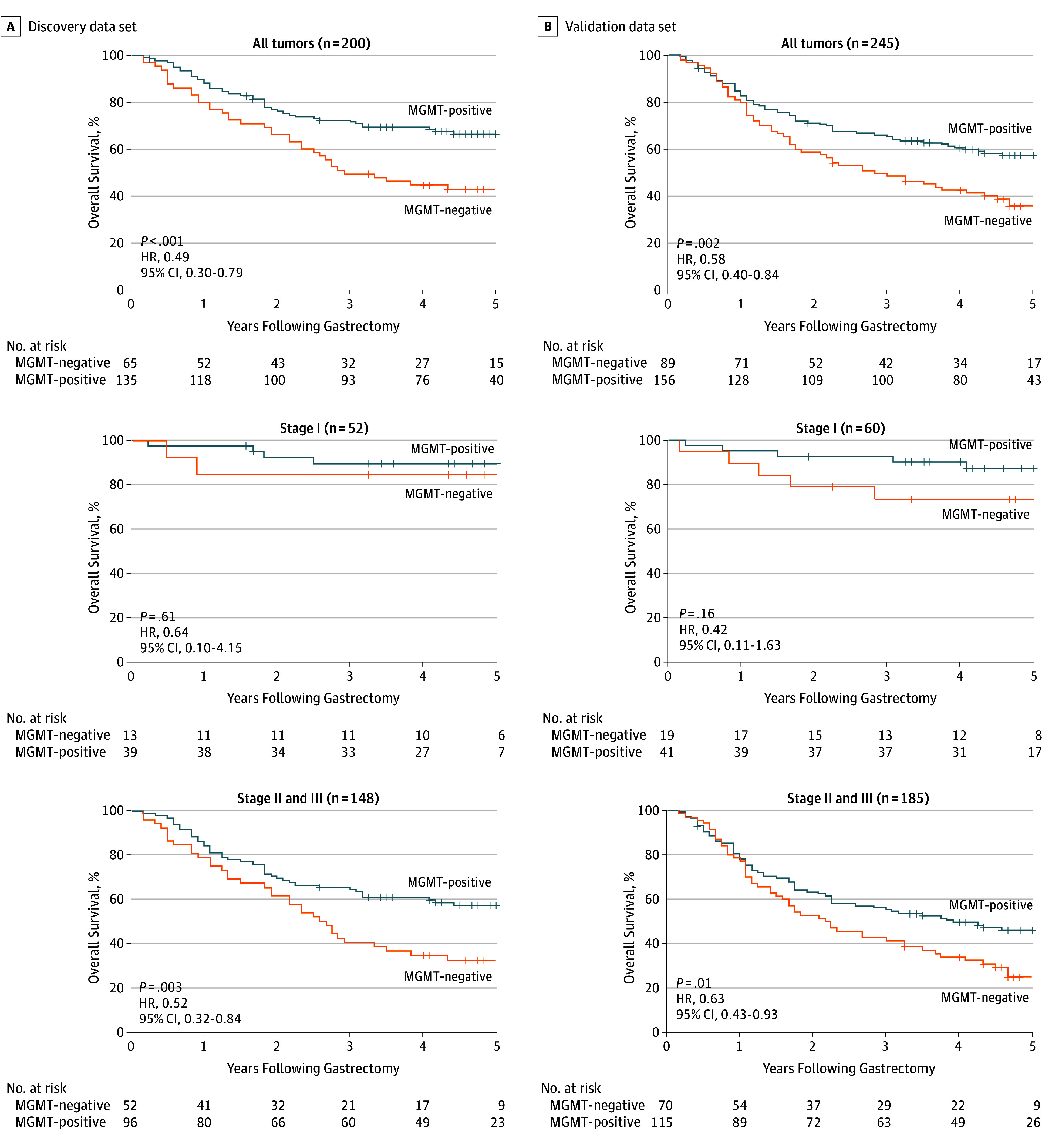
Kaplan-Meier analysis of overall survival was performed on the basis of MGMT expression in patients with resectable gastric cancer. The association between MGMT expression and overall survival was investigated in a discovery data set (A) and a validation data set (B). In the discovery data set, patients with stage II or III disease had better overall survival if the tumor was MGMT-positive (hazard ratio [HR], 0.52; 95% CI, 0.32-0.84; P = .003), whereas in the stage I subgroup, no substantial difference in the overall survival was observed between patients with MGMT-positive tumors and those with MGMT-negative tumors (HR, 0.64; 95% CI, 0.10-4.15; P = .61). The result of the validation data set was consistent with that of the discovery data set.
Cox Proportional Hazards Regression Analysis
After the tests of proportional hazards assumption (eTable 2 in the Supplement), we performed a multivariate Cox regression analysis, including age, sex, differentiation, Lauren classification, TNM stage, and MGMT expression. In the discovery data set, MGMT expression (HR, 0.61; 95% CI, 0.40-0.96; P = .03) and TNM stage (HR, 2.76; 95% CI, 1.88-4.04; P < .001) were identified as independent prognostic factors for the OS of resectable gastric cancer patients (Table 1), a finding confirmed by the MGMT expression (HR, 0.68; 95% CI, 0.48-0.98; P = .04) and TNM stage (HR, 2.58; 95% CI, 1.91-3.49; P < .001) in the validation data set (Table 1). Hence, our findings suggest that positive expression of MGMT could be a reliable, independent, favorable molecular prognosticator of OS for patients with resectable gastric cancer.
Table 1. Multivariate Cox Regression Analysis for Overall Survival.
| Factors | Discovery Data Set | Validation Data Set | ||
|---|---|---|---|---|
| HR (95% Cl) | P Value | HR (95% Cl) | P Value | |
| Age, modeled as a continuous variable | 1.00 (0.99-1.02) | .67 | 1.02 (1.01-1.04) | .005a |
| Sex | ||||
| Women | 1 [Reference] | .11 | 1 [Reference] | .15 |
| Men | 0.67 (0.41-1.09) | 0.76 (0.52-1.11) | ||
| Differentiation | ||||
| Differentiated | 1 [Reference] | .80 | 1 [Reference] | .92 |
| Undifferentiated | 1.08 (0.62-1.87) | 1.02 (0.65-1.61) | ||
| Lauren classification | ||||
| Intestinal type | 1 [Reference] | .83 | 1 [Reference] | .40 |
| Diffuse type | 0.95 (0.58-1.54) | 1.17 (0.81-1.69) | ||
| TNM stage according to increase in stage | 2.76 (1.88-4.04) | <.001a | 2.58 (1.91-3.49) | <.001a |
| MGMT expression | ||||
| Negative | 1 [Reference] | .03a | 1 [Reference] | .04a |
| Positive | 0.61 (0.40-0.96) | 0.68 (0.48-0.98) | ||
Abbreviations: HR, hazard ratio; MGMT, O6-methylguanine-DNA methyltransferase.
Shows statistical significance.
Prognostic Power for MGMT Expression
After MGMT expression and TNM stage were proved to be 2 independent prognosticators, we sought to assess whether the incorporation of MGMT into the current TNM staging system would improve the prognostic power of MGMT. Prognostic capability was compared with the use of time-dependent AUC. As shown in eFigure 1 in the Supplement, the AUC of MGMT was lower than the AUC of the TNM stage. However, the incorporation of MGMT into TNM stage showed better prognostic power in OS than TNM stage or MGMT alone. To build a more informative prognostic model, we established a nomogram that includes age, Lauren classification, and differentiation, besides TNM stage and MGMT (eFigure 2 in the Supplement). The prognostic nomogram performed well in postoperative OS for patients with gastric cancer, and the accuracy of this forecast was supported by the C-index (0.70) and the calibration curves (eFigure 3 in the Supplement).
MGMT Expression and Fluorouracil-Based Adjuvant Chemotherapy Benefit
Furthermore, we investigated whether patients with MGMT-positive or MGMT-negative tumors could benefit from postoperative adjuvant chemotherapy. A test for an interaction between MGMT expression and adjuvant chemotherapy indicated that, in stage II disease, the benefit from fluorouracil-based adjuvant chemotherapy was superior among patients who had MGMT-positive (HR, 0.35; 95% CI, 0.13-0.95; P = .007 for interaction; Table 2) tumors than among those with MGMT-negative tumors. However, the interaction test in stage III disease did not show significant results between patients who with MGMT-positive tumors (HR, 0.28; 95% CI, 0.17-0.46; P = .08 for interaction; Table 2) and those with MGMT-negative tumors. The corresponding Kaplan-Meier survival curves for patients with stage II or stage III disease, which comprehensively compared positive with negative MGMT expression by treatment, are reported in eFigure 4 in the Supplement. Consequently, these results suggest that patients with stage II, MGMT-positive tumors and all patients with stage III disease could benefit from fluorouracil-based adjuvant chemotherapy.
Table 2. Treatment Interaction With MGMT Expression for Overall Survivala.
| MGMT Expression | Patients With Stage II Disease (n = 104) |
Patients With Stage III Disease (n = 229) |
||||||
|---|---|---|---|---|---|---|---|---|
| With ACT | Without ACT | With ACT vs Without ACT, HR (95% CI) | P Value for Interaction | With ACT | Without ACT | With ACT vs Without ACT, HR (95% CI) | P Value for Interaction | |
| Negative | 18 | 11 | 0.71 (0.27-1.92) | .007b | 70 | 23 | 0.35 (0.21-0.59) | .08 |
| Positive | 48 | 27 | 0.35 (0.13-0.95) | 112 | 24 | 0.28 (0.17-0.46) | ||
Abbreviations: ACT, adjuvant chemotherapy; HR, hazard ratio; MGMT, O6-methylguanine-DNA methyltransferase.
Estimated from a Cox proportional hazards model on the combined data set of 333 patients with stage II or III gastric cancer.
Shows statistical significance.
Discussion
Functioning as a DNA repair enzyme, MGMT can rapidly repair adducts at the O6 position of guanine and act with the DNA mismatch-repair (MMR) system.26 Previous studies were mainly focused on the role MGMT played in neural tumors. The silencing of the MGMT gene or the methylation of the MGMT promoter in either glioma or glioblastoma was proved to be an adverse prognosticator27,28 and is reliably correlated with tumor responsiveness to alkylating agent–based adjuvant chemotherapy.7,26,29 Several studies have shown that the deficiency of MGMT can increase the sensitivity of high-grade glioma to alkylating agent–based adjuvant chemotherapy,7,30,31,32 but many tumors with a low MGMT expression level are nevertheless chemoresistant.33,34
As a kind of antimetabolite drug, fluorouracil is generally applied as a first-line postoperative adjuvant treatment for patients with advanced gastric cancer.4 The antineoplastic mechanism of fluorouracil is mainly dependent on inhibiting crucial biosynthetic processes or incorporating itself into macromolecules as DNA or RNA to interfere with their normal biological function.35 To our knowledge, no study has reported the explicit association between MGMT expression and benefit from fluorouracil-based adjuvant chemotherapy in gastric cancer. However, it was previously proved that the MMR system is involved in the effectiveness of fluorouracil. In colon cancer, MMR-deficient cancer cell lines losing expression of hMLH1 (OMIM U07418.1) were reported to show significantly more tolerance to fluorouracil than the chromosomal transfer–established, MMR-proficient HCT116+chr3 cells, indicating that the deficiency of DNA MMR might act as a key mechanism for tumor resistance to fluorouracil.36 In addition, defective DNA MMR can make tumor cells tolerant to mismatched base pairing, and mutations in p53 as well as overexpression of antiapoptotic protein such as Bcl-2 or Bcl-XL may ultimately result in resistance to proapoptotic signals.37 Consequently, fluorouracil responsiveness might be much more complicated than initially thought.
This fluorouracil-based adjuvant chemotherapy research recruited patients stratified as having late-stage (stage II or III) gastric cancer. To prevent excessive toxic effects, we believed it was important to identify patients who might benefit the most from postoperative adjuvant chemotherapy. Consequently, we further investigated the association between the clinical outcomes of patients with MGMT-negative or MGMT-positive cancer and fluorouracil-based adjuvant chemotherapy. As a result, patients with stage II, MGMT-positive gastric cancer enjoyed a significant survival benefit from fluorouracil-based adjuvant chemotherapy, whereas patients with stage II, MGMT-negative gastric cancer did not. This result suggests that MGMT could be an important factor for anticipating the responsiveness of fluorouracil-based chemotherapy in stage II disease. This finding will be of much use for better selection and treatment of patients recommended to receive adjuvant chemotherapy.
Limitations
This study has several limitations. It is retrospective in nature, and the number of patients who received adjuvant chemotherapy is relatively small. The original data are from a total patient cohort of 496 patients from Zhongshan Hospital, Fudan University. However, as described in the Methods section, the findings may not be generalizable owing to patient sample selection, inclusion, and exclusion and problems with initial tissue microarray construction and data loss. In addition, the full chemotherapy details might not be available for the entire cohorts. Therefore, validation of these results still require a prospective, larger, multicentered randomized trial.
Conclusions
This study clarified that positive expression of MGMT in gastric cancer was an independent, favorable prognostic factor. Combining MGMT expression with the current TNM staging system could improve MGMT’s prognostic capacity. Positive expression of MGMT also identified a subgroup of patients with stage II disease who could benefit from fluorouracil-based adjuvant chemotherapy. These findings pave the way for individual chemotherapy in patients with gastric cancer, but these results need to be validated given the retrospective and exploratory design of our study. We advocate for the confirmation of these findings within the framework of randomized clinical trials associated with genomic DNA sequencing studies.
eMethods 1. Construction of Tissue Microarray
eMethods 2. Immunohistochemistry
eTable 1. Relationship Between MGMT Expression and Clinical Characteristics
eTable 2. Tests of Proportional Hazards Assumption
eFigure 1. Time-Dependent AUC Analysis to Show Prognostic Power of MGMT Expression and TNM Stage
eFigure 2. Gastric Cancer Survival Nomogram
eFigure 3. Calibration Plots for the Survival Nomogram
eFigure 4. Association Between MGMT Expression and Benefit from 5-FU–Based Adjuvant Chemotherapy
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333(1):32-41. [DOI] [PubMed] [Google Scholar]
- 4.De Vita F, Orditura M, Matano E, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92(9):1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med. 1994;330(16):1136-1142. [DOI] [PubMed] [Google Scholar]
- 6.Smyth EC, Fassan M, Cunningham D, et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy trial. J Clin Oncol. 2016;34(23):2721-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350-1354. [DOI] [PubMed] [Google Scholar]
- 8.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793-797. [PubMed] [Google Scholar]
- 9.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50(19):6119-6129. [PubMed] [Google Scholar]
- 10.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16(4):168-174. [DOI] [PubMed] [Google Scholar]
- 11.Qian XC, Brent TP. Methylation hot spots in the 5′ flanking region denote silencing of the O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1997;57(17):3672-3677. [PubMed] [Google Scholar]
- 12.Rosas SLB, Koch W, da Costa Carvalho MG, et al. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61(3):939-942. [PubMed] [Google Scholar]
- 13.Cao Y, Zhang H, Liu H, et al. Glycoprotein 130 is associated with adverse postoperative clinical outcomes of patients with late-stage non-metastatic gastric cancer. Sci Rep. 2016;6:38364. doi: 10.1038/srep38364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Liu H, Zhang H, et al. CXC chemokine receptor 1 predicts postoperative prognosis and chemotherapeutic benefits for TNM II and III resectable gastric cancer patients. Oncotarget. 2017;8(12):20328-20339. doi: 10.18632/oncotarget.12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C, Liu H, Zhang H, et al. Interleukin-13 receptor α2 is associated with poor prognosis in patients with gastric cancer after gastrectomy. Oncotarget. 2016;7(31):49281-49288. doi: 10.18632/oncotarget.10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Zhang H, Liu H, et al. High expression of C-C chemokine receptor 2 associates with poor overall survival in gastric cancer patients after surgical resection. Oncotarget. 2016;7(17):23909-23918. doi: 10.18632/oncotarget.8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Shen Z, Wang Z, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep. 2016;6:21319. doi: 10.1038/srep21319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Zhang H, Shen Z, et al. Increased expression of CSF-1 associates with poor prognosis of patients with gastric cancer undergoing gastrectomy. Medicine (Baltimore). 2016;95(9):e2675. doi: 10.1097/MD.0000000000002675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Liu H, Zhang H, et al. Galectin-8 is associated with recurrence and survival of patients with non-metastatic gastric cancer after surgery. Tumour Biol. 2016;37(9):12635-12642. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Liu H, Zhang H, et al. Decreased expression of Siglec-8 associates with poor prognosis in patients with gastric cancer after surgical resection. Tumour Biol. 2016;37(8):10883-10891. doi: 10.1007/s13277-016-4859-7 [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Shen Z, Wang X, et al. Increased expression of C-C motif ligand 2 associates with poor prognosis in patients with gastric cancer after gastrectomy. Tumour Biol. 2016;37(3):3285-3293. doi: 10.1007/s13277-015-4092-9 [DOI] [PubMed] [Google Scholar]
- 22.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called Intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [DOI] [PubMed] [Google Scholar]
- 23.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077-3079. [DOI] [PubMed] [Google Scholar]
- 24.Shou Z-X, Jin X, Zhao Z-S. Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann Surg. 2012;256(6):1014-1022. [DOI] [PubMed] [Google Scholar]
- 25.Clifford JL, Menter DG, Yang X, et al. Expression of protein mediators of type I interferon signaling in human squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2000;9(9):993-997. [PubMed] [Google Scholar]
- 26.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 27.Gao D, Herman JG, Guo M. The clinical value of aberrant epigenetic changes of DNA damage repair genes in human cancer. Oncotarget. 2016;7(24):37331-37346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanemoto M, Shirahata M, Nakauma A, et al. Prognostic prediction of glioblastoma by quantitative assessment of the methylation status of the entire MGMT promoter region. BMC Cancer. 2014;14:641-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Nifterik KA, van den Berg J, van der Meide WF, et al. Absence of the MGMT protein as well as methylation of the MGMT promoter predict the sensitivity for temozolomide. Br J Cancer. 2010;103(1):29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan ME, Moschel RC, Pegg AE. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990;87(14):5368-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolan ME, Pegg AE. O6-benzylguanine and its role in chemotherapy. Clin Cancer Res. 1997;3(6):837-847. [PubMed] [Google Scholar]
- 32.Friedman HS, Kokkinakis DM, Pluda J, et al. Phase I trial of O6-benzylguanine for patients undergoing surgery for malignant glioma. J Clin Oncol. 1998;16(11):3570-3575. [DOI] [PubMed] [Google Scholar]
- 33.Friedman HS, McLendon RE, Kerby T, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol. 1998;16(12):3851-3857. [DOI] [PubMed] [Google Scholar]
- 34.Belanich M, Randall T, Pastor MA, et al. Intracellular localization and intercellular heterogeneity of the human DNA repair protein O6-methylguanine-DNA methyltransferase. Cancer Chemother Pharmacol. 1996;37(6):547-555. [DOI] [PubMed] [Google Scholar]
- 35.Zoli W, Ulivi P, Tesei A, et al. Addition of 5-fluorouracil to doxorubicin-paclitaxel sequence increases caspase-dependent apoptosis in breast cancer cell lines. Breast Cancer Res. 2005;7(5):R681-R689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117(1):123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkaria JN, Kitange GJ, James CD, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14(10):2900-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Construction of Tissue Microarray
eMethods 2. Immunohistochemistry
eTable 1. Relationship Between MGMT Expression and Clinical Characteristics
eTable 2. Tests of Proportional Hazards Assumption
eFigure 1. Time-Dependent AUC Analysis to Show Prognostic Power of MGMT Expression and TNM Stage
eFigure 2. Gastric Cancer Survival Nomogram
eFigure 3. Calibration Plots for the Survival Nomogram
eFigure 4. Association Between MGMT Expression and Benefit from 5-FU–Based Adjuvant Chemotherapy