Abstract
Background
Pulmonary valve replacement (PVR) in patients with repaired tetralogy of Fallot (rTOF-TAP) is often based on cardiac MRI (CMR) right ventricular (RV) volumes indexed to body surface area (BSA). Weight extremes result in increased patient morbidity and affect indexed measurements. We hypothesized that patients with rTOF-TAP at extremes of weight have: 1) over- or underestimated indexed volumes and 2) altered parameters of cardiac function.
Methods
CMRs in patients with rTOF-TAP were retrospectively reviewed; analysis included right and left ventricular (LV) volumes and ejection fractions (EF) and peak global LV circumferential strain (ε cc) from myocardial tagged images. Indexed volumes were recalculated using ideal BSA. Weight categories were assigned: underweight, appropriate weight, overweight and obese. Linear regression models with weight category, spline of age and gender were created to assess the association of weight and parameters of volume and function.
Results
When RV volumes were corrected for ideal BSA, 11 (31%) additional overweight and obese patients met published criteria for PVR and 3 (38%) underweight patients no longer met criteria. Obese and overweight patients had larger absolute LV and RV diastolic volumes, but no difference in volumes indexed to ideal BSA. Modeling demonstrated no difference in LVEF or RVEF by weight categories but significant differences in global LV ε cc
Conclusions
Extremes of body weight may result in inappropriate timing of PVR. Extremes of weight lead to abnormalities in global LV ε cc. Although clinical implications of abnormal ε cc are unclear, these patients may be at higher risk for early ventricular dysfunction.
Keywords: Tetralogy of Fallot, Cardiac Magnetic Resonance, Obesity, Body Mass Index, Body Surface Area, Strain
INTRODUCTION
Tetralogy of Fallot (TOF) is the most common form of cyanotic congenital heart disease, occurring in approximately 0.04% of all live births, and repair often involves a trans-annular patch (TAP)[1]. Patients with repaired tetralogy of Fallot with trans-annular patch (rTOF-TAP) have subsequent pulmonary insufficiency and progressive right ventricular (RV) enlargement[2]. This progressive RV dilation may result in onset of exercise intolerance, arrhythmia, biventricular dysfunction and heart failure[3]. Surgical artificial pulmonary valve replacement (PVR) is performed to mitigate the effects of chronic pulmonary insufficiency, but timing of PVR has been intensely debated in the literature[4-9].
Surveillance CMR has been established as the optimal imaging modality of the RV[10]. Indications for PVR based on CMR have been published and these recommendations in part include body surface area (BSA)-indexed RV end diastolic volume (RVEDVi) >150ml/m2 or indexed RV end systolic volume (RVESVi) >80ml/m2[11,12]. Indexing of CMR volumes to BSA allows for comparison of various age and gender groups[13]. Extremes of weight can lead to an overestimation of RV volumes[14], but the effects of being underweight or frail have not been evaluated.
Obesity is a severe health epidemic that has not spared children and adults with congenital heart disease[15,16]. Excess body weight has numerous deleterious effects on health, in particular on cardiac function[17,18]. Underweight pediatric patients are also at risk for increased morbidity and have been associated with higher risk for post-surgical complications, longer length of stay and increased cost of hospitalization[19]. Poor nutrition has been associated with increased morbidity during initial surgical repair of Tetralogy of Fallot[20,21]. Insufficient post-operative weight gain has also been associated with increased risk of mortality following congenital heart surgery[22]. Adults with low BMI post-revascularization procedures have increased risk for CV mortality[23] and adults with low or high BMI have increased risk for post-surgical CV complications and mortality[24]. We hypothesized that: 1) underweight, overweight, and obese patients with rTOF-TAP have over- or underestimated indexed ventricular volumes when compared to volumes indexed to ideal BSA and 2) these patients have altered parameters of cardiac function compared to weight appropriate patients.
MATERIALS AND METHODS
Study population demographics
This was a retrospective study approved by the Institutional Review Board and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The CMR database was reviewed from 2009-2013 and patients with TOF were identified. Only patients with previous rTOF-TAP were included in the study. Patients with rTOF-TAP after PVR, valve-sparing TOF repair, TOF with RV-PA conduit repair, original diagnosis of TOF with pulmonary atresia, TOF with absent pulmonary valve syndrome, critical pulmonary stenosis or pulmonary atresia were excluded from the study. A total of 165 patients were identified and 86 met inclusion and exclusion criteria for the study cohort.
Calculation of Body Mass Index and Weight Group Assignments
BMI was calculated as weight in kilograms divided by square of height in meters. Patients were grouped into four weight categories; underweight, appropriate weight, overweight and obese, based on Centers for Disease Control and Prevention (CDC), National Heart, Lung and Blood Institute (NHLBI), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established guidelines[25]. Patients aged 20.0 years and younger were grouped using the following categories based on standard gender-based CDC growth charts: 1) underweight, BMI less than 5th percentile for age, 2) appropriate weight, BMI between 5th and 85th percentile for age, 3) overweight, BMI between 85th and 95th percentile for age, and 4) obese, BMI greater than 95th percentile for age. Patients aged 20.1 years and older were grouped using the following categories: 1) underweight, BMI less than 18.5 kg/m2, 2) appropriate weight, BMI between 18.5 kg/m2 and 24.9 kg/m2, 3) overweight, BMI between 25.0 and 29.9 kg/m2, and 4) obese, BMI greater than 30 kg/m2.
Calculation of Ideal Body Weight
Patients were assigned corrected ideal body weight (IBW) using the standard gender-based formulas to correct for gender-specific differences in body habitus.
For patients of all ages in appropriate weight group, no correction was performed. For male patients aged 20.1 years and older, in underweight, overweight, and obese groups, IBW was corrected and recalculated using the Devine formula for males[26]: IBW (kg) = 50 kg + 2.3 kg * (Height (inches) − 60). For female patients aged 20.1 years and older, in underweight, overweight, and obese groups, IBW was corrected and recalculated using the Devine formula for females[26]: IBW (kg) = 45.5 kg + 2.3 kg * (Height (inches) − 60).
For patients aged 20.0 years and younger who were underweight, overweight, or obese, ideal BMI was determined by calculation of the 50th percentile gender specific BMI for age. To calculate the IBW, the ideal BMI was multiplied by the square of the height (m2). This method has been shown to be most accurate method for calculating IBW in children at extremes of body weight[27].
Calculation of Body Surface Area
For patients of all ages in appropriate weight groups, the actual body surface area (BSA) was calculated using the Haycock formula[28]: BSA = [0.024265 × height (cm)0.3964 × weight (kg)0.5378]. For patients of all ages in underweight, overweight, and obese weight groups, ideal BSA was calculated using the Haycock formula while substituting the patient’s ideal body weight in place of actual body weight.
CMR Acquisition
Images were obtained on either a 1.5 Tesla Siemens Avanto (Siemens Healthcare Sector, Erlangen, Germany) or a 1.5 Tesla Philips Intera (Philips Medical Systems, Best, The Netherlands). Functional imaging was performed as previously described using balanced steady state free-precession images in a short axis stack[29]. Myocardial tagging was performed in the short axis at the level of the papillary muscles using a segmented k-space fast gradient echo sequence with ECG triggering. Grid tagging was performed with a spacing of 8 mm and 8-10 phases (Philips) or 9-13 phases (Siemens). Typical imaging parameters included: slice thickness 6-8 mm, field of view 340 mm × 340 mm, matrix size 256 × 192, and minimum echo time and repetition time. The sequences were breath-holds and parallel imaging with GRAPPA (Siemens) and SENSE (Philips) with an acceleration factor of two was used.
CMR Analysis
Right and left ventricular volumes and ejection fractions were calculated with manual contouring of the endocardial borders in end-diastole and end-systole using the Leonardo Workstation (Siemens Healthcare Sector, Erlangen, Germany) or the Extended MR WorkSpace (Philips Medical Systems, Best, The Netherlands). These measurements were indexed by dividing each value by the actual BSA and ideal BSA for comparison. Regurgitant volumes and regurgitant fractions were calculated from phase contrast imaging in the main pulmonary artery.
Strain Analysis
Analysis of myocardial tagged images was performed using harmonic phase (HARP) methodology (Diagnosoft Inc., Morrisville, NC). One reader (SS) performed the analysis blinded to BMI and other pertinent clinical data. A mesh was created by manually contouring the endocardial and epicardial borders at end-systole. The software then calculated the peak global and segmental circumferential strain (ε cc) values; segmental values were calculated in the 6 segments at the mid portion of the LV using the standard 17 segment model[30]. Peak RV ε cc was calculated using HARP analysis of myocardial tagged images in three regions of RV base, body and outflow as previously described[31]. Of 86 study cohort patients, 15 patients were excluded from strain analysis due to lack of myocardial tagging during original CMR data collection.
Calculating Ideal Ventricular Volumes
The ideal BSA was used to recalculate RVEDV, RVESV, LVEDV, and LVESV in all patients who fell in the underweight, overweight, and obese categories. Patients who crossed CMR cut-offs were identified.
Statistical Analysis
Four groups of patients (Underweight, Appropriate Weight, Overweight, and Obese) were compared using a Kruskal-Wallis test on the outcomes of interest. Linear regression models with BMI category, spline of age and gender were fitted to assess the association of BMI category with the outcomes of interest adjusting for age and gender. Predicted values were calculated and plotted. Statistical analysis was performed using R studio 3.0.2 (online at http://www.rstudio.com/). Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt University[32].
RESULTS
Demographics
In the cohort of 86 patients, 38% were male (Table 1). Surgical palliation was performed prior to definitive repair (VSD closure and TAP) in 24% of patients; palliations included a classic Blalock-Taussig shunt, modified Blalock-Taussig shunt, Waterston shunt or other systemic to pulmonary shunt. The median age at time of definitive repair was 7 months (range 0.3-120 months) and the median age at CMR was 17.5 years (range 3.3-54.8 years) (Table 1).
Table 1. Study Cohort Demographics.
| n=86 | |
|---|---|
| Gender (male) | 38% (n=33) |
| Prior surgical palliation | 24% (n=21) |
| Median age at Surgical Repair (mo) | 7 (5, 16)a |
| Age at CMR (yr) | 17.5 (13, 23) |
| Height (cm) | 155.3 ± 17.4 |
| Weight (kg) | 58.6 ± 22.1 |
| Body mass index (kg/m2) | 23.5 ± 6.2 |
| Body surface area (m2) | 1.6 ± 0.4 |
|
| |
| Absolute RVEDV (ml) | 214 (180-251) |
| RVEDVi (ml/m2) | 134 (119-163) |
| Absolute RVESV (ml) | 101 (77-125) |
| RVESVi (ml/m2) | 65 (51-83) |
| RVEF (%) | 53 (47-58) |
| Absolute LVEDV (ml) | 100 (79-122) |
| LVEDVi (ml/m2) | 65 (57-73) |
| Absolute LVESV (ml) | 37 (25-48) |
| LVESVi (ml/m2) | 23 (18-30) |
| LVEF (%) | 63 (58-69) |
Median (interquartile range)
Mean ± standard deviation
Corrected Volumes
In order to evaluate the effect of weight on CMR defined criteria, four BMI-based groups were created (Table 2). BMI-based grouping demonstrated eight (9%) patients in the underweight group, 43 (50%) patients in the appropriate weight group, 23 (27%) patients in the overweight group and 12 (14%) patients in the obese group. There was no significant difference in gender within weight groups, though there was a significant difference in age between the groups.
Table 2. Weight groups.
| Underweight N = 8 | Appropriate Weight N = 43 | Overweight N = 23 | Obese N = 12 | P-Value | |
|---|---|---|---|---|---|
| Age (years) | 11.4 (10.6-13.3) | 17.7 (12.9-19.9) | 22 (16.6-36.5) | 15.4 (13.3-18.1) | 0.002a |
| Male % | 25% (2) | 33% (14) | 39% (9) | 67% (8) | 0.152b |
|
| |||||
| Absolute RVEDV (ml) | 180 (122-220) | 200 (180-236) | 222 (187-250) | 258 (212-275) | 0.024a |
| RVEDVi (ml/m2) | 160 (147-179) | 137 (120-168) | 129 (120-144) | 128 (101-153) | 0.160a |
| Ideal RVEDVi (ml/m2) | 141 (125-153) | 137 (120-168) | 148 (135-163) | 160 (136-199) | 0.207a |
| Absolute RVESV (ml) | 93 (51-111) | 101 (76-120) | 100 (79-129) | 121 (100-150) | 0.249a |
| RVESVi (ml/m2) | 77 (61-100) | 66 (54-85) | 61 (47-74) | 61 (51-79) | 0.397a |
| Ideal RVESVi (ml/m2) | 67 (52-86) | 66 (54-85) | 72 (53-80) | 75 (63-102) | 0.645a |
| RVEF (%) | 58 (45-58) | 52 (47-56) | 53 (47-58) | 52 (48-60) | 0.938a |
| RV Regurgitant Fraction (%) | 49 (44-55) | 44 (37-51) | 37 (31-45) | 43 (40-51) | 0.115a |
| RV Regurgitant Volume (ml) | 29 (21-52) | 40 (30-52) | 43 (31-57) | 45 (40-52) | 0.333a |
|
| |||||
| Absolute LVEDV (ml) | 63 (60-92) | 94 (80-104) | 118 (101-136) | 115 (89-122) | 0.007a |
| LVEDVi (ml/m2) | 70 (61-78) | 65 (57-74) | 68 (58-73) | 59 (44-70) | 0.263a |
| Ideal LVEDVi (ml/m2) | 61 (53-68) | 65 (57-74) | 74 (66-81) | 70 (56-87) | 0.063a |
| Absolute LVESV (ml) | 28 (19-36) | 35 (24-42) | 40 (32-51) | 44 (32-50) | 0.103a |
| LVESVi (ml/m2) | 27 (20-32) | 24 (18-29) | 22 (19-29) | 22 (17-27) | 0.706a |
| Ideal LVESVi (ml/m2) | 23 (17-28) | 24 (18-29) | 26 (21-32) | 28 (22-33) | 0.385a |
| LVEF (%) | 63 (55-71) | 63 (59-71) | 63 (60-68) | 63 (56-67) | 0.782a |
RVEDV – right ventricular end diastolic volume. RVEDVi – indexed right ventricular end diastolic volume. RVESV – right ventricular end systolic volume. RVESVi – indexed right ventricular end systolic volume. RVEF – right ventricular ejection fraction. LVEDV – left ventricular end diastolic volume. LVEDVi – indexed left ventricular end diastolic volume. LVESV – left ventricular end systolic volume. LVESVi – indexed left ventricular end systolic volume. LVEF – left ventricular ejection fraction.
Kruskal-Wallis test
Pearson Test.
To assess clinical implications of correcting indexed volumes with ideal BSA, we recalculated ideal BMI and ideal BSA in underweight, overweight and obese patients within our cohort. As would be expected, underweight patients had corrected ideal RVEDVi and ideal RVESVi that were decreased from original non-ideal indexed values (Figure 1 A, B). In the underweight group, six patients (75%) met published RVEDVi and four patients (50%) met published RVESVi referral criteria for PVR, respectively. When ideal RVEDVi was recalculated in this group, four patients (50%) no longer met PVR referral criteria for RVEDVi and one patient (13%) no longer met RVESVi referral criteria (Table 3). In total, three (38%) underweight patients no longer met CMR volumetric criteria for PVR referral when corrected for ideal BSA.
Figure 1. Effect of Ideal BSA Calculations.
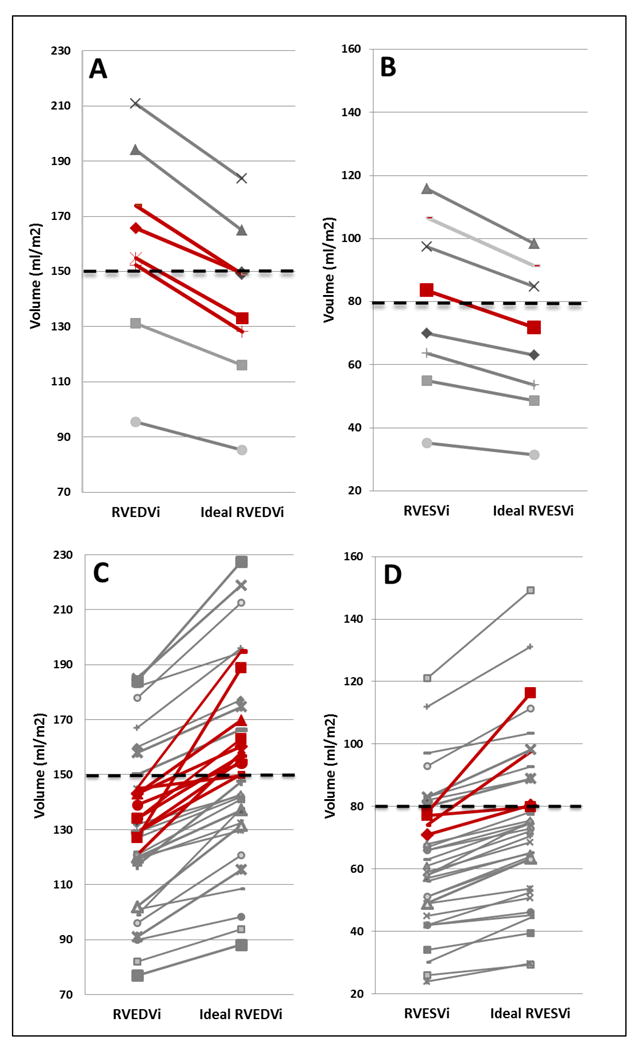
RVEDVi (Figure 1A) and RVESVi (Figure 1B) in underweight patients with subsequent change in RVEDVi and RVESVi after indexing to ideal BSA (ideal RVEDVi, ideal RVESVi). RVEDVi (Figure 1C) and RVESVi (Figure 1D) in overweight and obese patients with subsequent change in RVEDVi and RVESVi after indexing to ideal BSA (ideal RVEDVi, ideal RVESVi). Red lines represent patients who cross threshold for pulmonary valve replacement by ideal RVEDV or ideal RVESVi criteria.
Table 3. Percent Meeting pulmonary valve replacement (PVR) Criteria.
| PVR Criterion | Underweight N = 8 | Appropriate Weight N = 43 | Overweight N = 23 | Obese N = 12 | Overweight + Obese N = 35 | Total N = 86 |
|---|---|---|---|---|---|---|
| RVEDVi | 75 (6) | 42 (18) | 22 (5) | 25 (3) | 23 (8) | 37 (32) |
| Ideal RVEDVi | 25 (2) | 42 (18) | 48 (11) | 67 (8) | 54 (19) | 44 (38) |
| RV:LV Ratio | 63 (5) | 65 (28) | 43 (10) | 75 (9) | 54 (19) | 60 (52) |
|
| ||||||
| RVESVi | 50 (4) | 33 (14) | 22 (5) | 25 (3) | 23 (8) | 30 (26) |
| Ideal RVESVi | 38 (3) | 33 (14) | 30 (7) | 42 (5) | 34 (12) | 33 (28) |
| RVEF | 38 (3) | 21 (9) | 22 (5) | 17 (2) | 20 (7) | 22 (19) |
| LVEF | 25 (2) | 16 (7) | 13 (3) | 17 (2) | 14 (5) | 17 (15) |
Values represent percent of each body mass index category. Numbers in parenthesis are numbers of patients above or below the following thresholds: indexed right ventricular end diastolic volume (RVEDVi) and ideal RVEDVI >150 ml/m2, indexed right ventricular end systolic volume (RVESVi) and ideal RVESVi >80 ml/m2, right ventricular ejection fraction (RVEF) <47%, left ventricular ejection fraction (LVEF) <55%, RV:LV ratio >2.
Similarly, all overweight and obese patients had corrected ideal RVEDVi and ideal RVESVi that were increased from original non-ideal indexed values (Figure 1 C, D). When ideal RVEDVi was recalculated, an additional six overweight (26%) and five obese (42%) patients met published RVEDVi criteria for PVR referral (Table 3). When ideal RVESVi was calculated, two overweight (9%) and two obese (17%) patients who had previously met published indexed RVEDVi criteria also met indexed RVESVi criteria for PVR referral (Table 3). In total, an additional 13 (31%) overweight and obese patients met CMR volumetric criteria for PVR referral when corrected for ideal BSA.
When using the RVEDV:LVEDV ratio to determine candidacy for PVR, nine obese (75%) and ten overweight (43%) patients met volumetric ratio for PVR referral. Additionally, when the ratio criteria are applied to underweight and appropriate weight patients in our cohort, five underweight (63%) and 28 appropriate weight (65%) patients would also meet criteria for PVR. Using the RVEDV:LVEDV, 52 patients (60% of the total study cohort) meet PVR referral criteria.
Strain Analysis
Univariate analysis demonstrated a significant difference between weight groups for LV global ε cc (p = 0.023) and ε cc in the anteroseptal and inferior segments (p = 0.005 and p = 0.027, respectively). There was no significant difference in LVEF or RV ε cc parameters between groups using univariate analysis. When adjusted for age and gender there was a significant association between BMI category and LV global ε cc (p = 0.022) as well as LV ε cc in the inferior segment (p = 0.04) (Figure 2). These differences were driven by the difference in ε cc between obese and appropriate weight patients. Notably, there was no significant association between LVEF and BMI (p = 0.41) (Figure 2).
Figure 2. Analysis of Pertinent Outcomes of Interest with Associated Weight Classes.
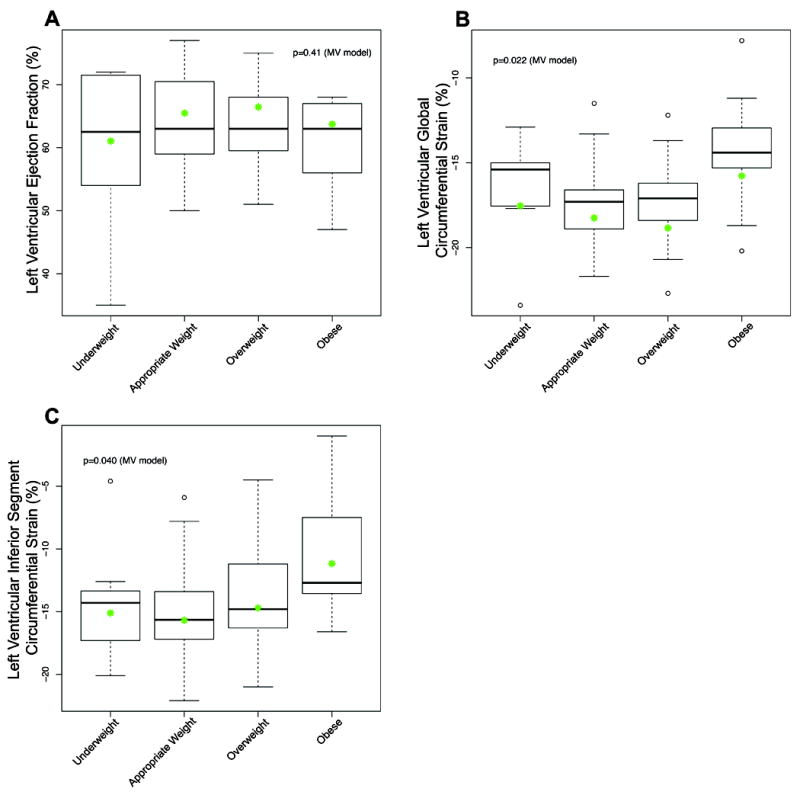
A. Left ventricular ejection fraction, B. Left ventricular global circumferential strain, C. Left ventricular inferior segment strain. Raw data are represented as box plots. The multivariate model (MV) corrected for gender and age in the association between weight category and outcomes of interest; predicted mean data are shown as green dots, assuming an age of 17.5 and female gender.
DISCUSSION
Our study demonstrates the following novel findings:
Extremes of body weight lead to significant under- and overestimation of RVEDVi and RVESVi in patients with rTOF-TAP relative to calculations based on ideal BSA.
Patients with rTOF-TAP have abnormalities in global myocardial strain associated with BMI weight categories.
This is the first study, to our knowledge, to evaluate underweight patients with rTOF-TAP. These findings are important because they impact timing of PVR. Moreover, these data demonstrate that obesity leads to subclinical LV dysfunction, suggesting that weight modification through diet and exercise could lead to improvements in outcomes in patients with rTOF-TAP.
Assessment of appropriate timing for PVR is integral to long-term outcomes. Performing surgery too early needlessly exposes patients to operative risks. Alternatively, delaying too long can theoretically lead to increased incidence of arrhythmia and ventricular dysfunction. Very few studies have evaluated the effect of weight on PVR timing, and none have evaluated underweight patients. We confirmed the findings of Maskatia et al, who found that correcting RV volumes in obese patients after tetralogy of Fallot repair using ideal BMI and BSA increases the number of patients who may require PVR[14]. Without this correction, referral for PVR may be delayed, potentially placing these patients at additional risk. We also demonstrated that using traditional methodology in underweight patients leads to overestimation of ventricular volumes. Given the increased surgical morbidity and mortality in underweight patients, avoiding unnecessary surgery in underweight patients is paramount.
Previous studies had suggested the use of the ratio of RVEDV:LVEDV in individuals with BSA larger than expected norms[10,11]. Using this methodology, 60% of our cohort would have met criteria for PVR, raising concern that this method of correction may be too aggressive. The RVEDV:LVEDV ratio applies a different methodology to assess PVR candidacy in obese and overweight patients than that currently used in appropriate weight patients. Interestingly, when we compared the two methods, we found that that they reclassified different obese and overweight patients as meeting criteria for PVR. While both methods are valid, we feel the use of ideal BSA to index RV volumes provides a more consistent method to identify patients for PVR across weight categories. Moreover, the ideal BSA is not affected by left ventricular dilation, a comorbidity that can be present in this patient population.
We were not able to rule out meaningful associations between BMI and RV ε cc. This may have been related to limitations in the methodology. Although this methodology has been reported previously[31], it is dependent on RV hypertrophy for analysis and therefore has not been validated in normal subjects. It is possible that assessment of RV longitudinal strain would be preferable or that changes in weight preferentially affect the LV myocardium.
This is the first study, to our knowledge, to find an association between BMI and global LV ε cc assessed by HARP in patients with rTOF-TAP. The assessment of myocardial strain has been reported separately in obese patients and in patients with rTOF. Abnormal myocardial strain is well described in obese patients by both CMR and echocardiography when compared with controls[33,34]. In the rTOF-TAP population, abnormal left ventricular strain and LV dysfunction has been shown[31]. Notably, we did not find an association between BMI and LVEF as was described by Maskatia et al[14]. However, our study agrees with more recent data suggesting no difference in LVEF between weight categories[35,36]. Our data also confirm the findings of Fogel et al of similar ventricular volumes across weight categories when using ideal BSA[35]. The association between global LV ε cc and BMI was not significant between underweight and appropriate weight groups, though this trend may reach significance with larger numbers. The LV ε cc in the inferior segment was also decreased in obese patients. It is unclear why this segment was most affected. It is possible that being underweight or overweight has a preferential effect on the inferior segment. It is also possible that this segment undergoes more injury through ventricular-ventricular interactions and RV enlargement[37].
Although the implications of abnormal LV ε cc for timing of PVR are unclear, abnormal LV ε cc suggests that obese patients may be at higher risk of developing LV dysfunction than their appropriate weight counterparts. These data reinforce the need for pediatric and adult congenital cardiologists to counsel patients to maintain healthy weights.
Limitations
This is a retrospective study in a small cohort of patients. However, the changes in indexed RV volumes in under and overweight patients are likely generalizable. The clinical implications of reduced myocardial strain are unclear and prospective studies with larger cohorts should be performed. Strain was only analyzed in the LV at the level of the papillary muscles; abnormalities in other LV segments would be missed with this methodology. We were unable to use absolute BMI as this cohort spanned pediatric and adult patients. In order to account for this, we categorized patients. Although not using a continuous variable in the analysis limits the power of the analysis, we felt it was necessary to account for different “normal ranges” of BMI depending on age and gender.
Conclusions
We demonstrate that extremes of body weight lead to both under- and overestimation of indexed RV volumes relative to calculations based on ideal BSA, significantly affecting the number of patients who meet criteria for PVR. Given the increased surgical morbidity and mortality in both underweight and overweight patients, accurate assessment of RV volumes is paramount. These values can be corrected using ideal BSA methodology. We also revealed a relationship between BMI and global LV ε cc. While the implications of decreased LV ε cc on PVR timing are unclear and require further evaluation, these data demonstrate that weight has an effect on LV function in patients with rTOF-TAP.
Acknowledgments
none
FUNDING:
The project was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Bethesda, MD) (Soslow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
ETHICAL APPROVAL: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW, Danielson GK. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329(9):593–599. doi: 10.1056/NEJM199308263290901. [DOI] [PubMed] [Google Scholar]
- 4.Quail MA, Frigiola A, Giardini A, Muthurangu V, Hughes M, Lurz P, Khambadkone S, Deanfield JE, Tsang V, Taylor AM. Impact of pulmonary valve replacement in tetralogy of Fallot with pulmonary regurgitation: a comparison of intervention and nonintervention. Ann Thorac Surg. 2012;94(5):1619–1626. doi: 10.1016/j.athoracsur.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 5.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116(5):545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 6.Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:11–22. doi: 10.1053/j.pcsu.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, Mist B, Walker F, van Doorn C, Bonhoeffer P, Taylor AM. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118(14 Suppl):S182–190. doi: 10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 8.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95(6):779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Kim YM, Lee CH, Kwak JG, Park CS, Song JY, Shim WS, Choi EY, Lee SY, Baek JS. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol. 2012;60(11):1005–1014. doi: 10.1016/j.jacc.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 10.Geva T. Is MRI the preferred method for evaluating right ventricular size and function in patients with congenital heart disease?: MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ Cardiovasc Imaging. 2014;7(1):190–197. doi: 10.1161/CIRCIMAGING.113.000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geva T. Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation. 2013;128(17):1855–1857. doi: 10.1161/CIRCULATIONAHA.113.005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, del Nido P. Randomized trial of pulmonary valve replacement with and without right ventricular remodeling surgery. Circulation. 2010;122(11 Suppl):S201–208. doi: 10.1161/CIRCULATIONAHA.110.951178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang SA, Choe YH, Jang SY, Kim SM, Lee SC, Oh JK. Assessment of left and right ventricular parameters in healthy Korean volunteers using cardiac magnetic resonance imaging: change in ventricular volume and function based on age, gender and body surface area. Int J Cardiovasc Imaging. 2012;28(Suppl 2):141–147. doi: 10.1007/s10554-012-0150-1. [DOI] [PubMed] [Google Scholar]
- 14.Maskatia SA, Spinner JA, Nutting AC, Slesnick TC, Krishnamurthy R, Morris SA. Impact of obesity on ventricular size and function in children, adolescents and adults with Tetralogy of Fallot after initial repair. Am J Cardiol. 2013;112(4):594–598. doi: 10.1016/j.amjcard.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Pinto NM, Marino BS, Wernovsky G, de Ferranti SD, Walsh AZ, Laronde M, Hyland K, Dunn SO, Jr, Cohen MS. Obesity is a common comorbidity in children with congenital and acquired heart disease. Pediatrics. 2007;120(5):e1157–1164. doi: 10.1542/peds.2007-0306. [DOI] [PubMed] [Google Scholar]
- 16.Shustak RJ, McGuire SB, October TW, Phoon CK, Chun AJ. Prevalence of obesity among patients with congenital and acquired heart disease. Pediatr Cardiol. 2012;33(1):8–14. doi: 10.1007/s00246-011-0049-y. [DOI] [PubMed] [Google Scholar]
- 17.Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, Devereux RB. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47(11):2267–2273. doi: 10.1016/j.jacc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48(11):2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao J, Peng L, Li R, Chen Y, Li X, Mo B, Li X. Nutritional risk screening and its clinical significance in hospitalized children. Clin Nutr. 2014;33(3):432–436. doi: 10.1016/j.clnu.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Egbe AC, Mittnacht AJ, Nguyen K, Joashi U. Risk factors for morbidity in infants undergoing tetralogy of fallot repair. Ann Pediatr Cardiol. 2014;7(1):13–18. doi: 10.4103/0974-2069.126539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirsch RE, Glatz AC, Gaynor JW, Nicolson SC, Spray TL, Wernovsky G, Bird GL. Results of elective repair at 6 months or younger in 277 patients with tetralogy of Fallot: a 14-year experience at a single center. J Thorac Cardiovasc Surg. 2014;147(2):713–717. doi: 10.1016/j.jtcvs.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Eskedal LT, Hagemo PS, Seem E, Eskild A, Cvancarova M, Seiler S, Thaulow E. Impaired weight gain predicts risk of late death after surgery for congenital heart defects. Arch Dis Child. 2008;93(6):495–501. doi: 10.1136/adc.2007.126219. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Vallakati A, Einstein AJ, Lavie CJ, Arbab-Zadeh A, Lopez-Jimenez F, Mukherjee D, Lichstein E. Relationship of body mass index with total mortality, cardiovascular mortality, and myocardial infarction after coronary revascularization: evidence from a meta-analysis. Mayo Clin Proc. 2014;89(8):1080–1100. doi: 10.1016/j.mayocp.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Thornqvist C, Gislason GH, Kober L, Jensen PF, Torp-Pedersen C, Andersson C. Body mass index and risk of perioperative cardiovascular adverse events and mortality in 34,744 Danish patients undergoing hip or knee replacement. Acta Orthop. 2014;85(5):456–462. doi: 10.3109/17453674.2014.934184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 26.Devine BJ. Gentamicin Therapy. Drug Intel Clin Phar. 1974;8(11):650–655. [Google Scholar]
- 27.Phillips S, Edlbeck A, Kirby M, Goday P. Ideal body weight in children. Nutr Clin Pract. 2007;22(2):240–245. doi: 10.1177/0115426507022002240. [DOI] [PubMed] [Google Scholar]
- 28.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 29.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15(1):35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial S, Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 31.Khalaf A, Tani D, Tadros S, Madan S. Right- and left-ventricular strain evaluation in repaired pediatric Tetralogy of Fallot patients using magnetic resonance tagging. Pediatr Cardiol. 2013;34(5):1206–1211. doi: 10.1007/s00246-013-0631-6. [DOI] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black D, Bryant J, Peebles C, Davies L, Inskip H, Godfrey K, Vettukattil J, Hanson M. Increased regional deformation of the left ventricle in normal children with increased body mass index: implications for future cardiovascular health. Pediatr Cardiol. 2014;35(2):315–322. doi: 10.1007/s00246-013-0778-1. [DOI] [PubMed] [Google Scholar]
- 34.Rider OJ, Ajufo E, Ali MK, Petersen SE, Nethononda R, Francis JM, Neubauer S. Myocardial tissue phase mapping reveals impaired myocardial tissue velocities in obesity. Int J Cardiovasc Imaging. 2015;31(2):339–347. doi: 10.1007/s10554-014-0548-z. [DOI] [PubMed] [Google Scholar]
- 35.Fogel MA, Pawlowski T, Keller MS, Cohen MS, Goldmuntz E, Diaz L, Li C, Whitehead KK, Harris MA. The Cardiovascular Effects of Obesity on Ventricular Function and Mass in Patients after Tetralogy of Fallot Repair. J Pediatr. 2015;167(2):325–330 e321. doi: 10.1016/j.jpeds.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Briston DA, Sabanayagam A, Zaidi AN. Observations on obesity patterns in tetralogy of Fallot patients from childhood to adulthood. Cardiol Young. 2017;27(5):890–894. doi: 10.1017/S1047951116001530. [DOI] [PubMed] [Google Scholar]
- 37.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43(6):1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]


