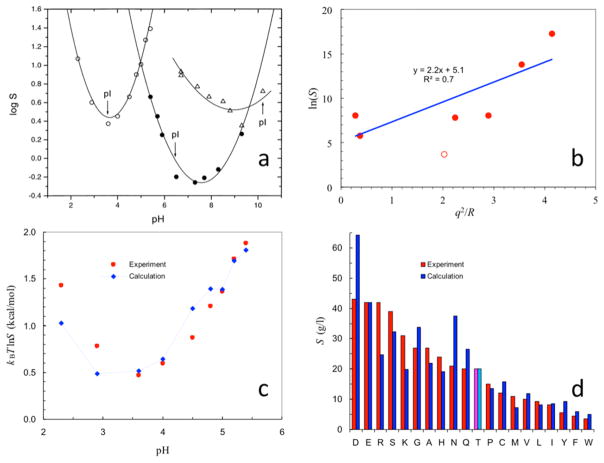
Figure 14.
Effects of pH, net charges, and mutations on protein solubility. (a) Dependence of solubility on pH for wild-type ribonuclease Sa and two charge mutants with very different isoelectric points (pI values; indicated by arrows). Reprinted with permission from ref 468. Copyright 2001 John Wiley & Sons Ltd. (b) Solubility data for seven proteins,473 linearly correlated with q2/R, according to eq 44a. Net charge q from ref 473; radius R estimated as 0.89MW1/3 Å, where MW denotes molecular weight in daltons. (c) Calculated pH dependence of wild-type ribonuclease Sa solubility. (d) Calculated results for ribonuclease Sa solubility when Thr76 is mutated into the other 19 types of amino acids. Panels c and d reprinted with permission from ref 27. Copyright 2008 Elsevier.