Figure 9.
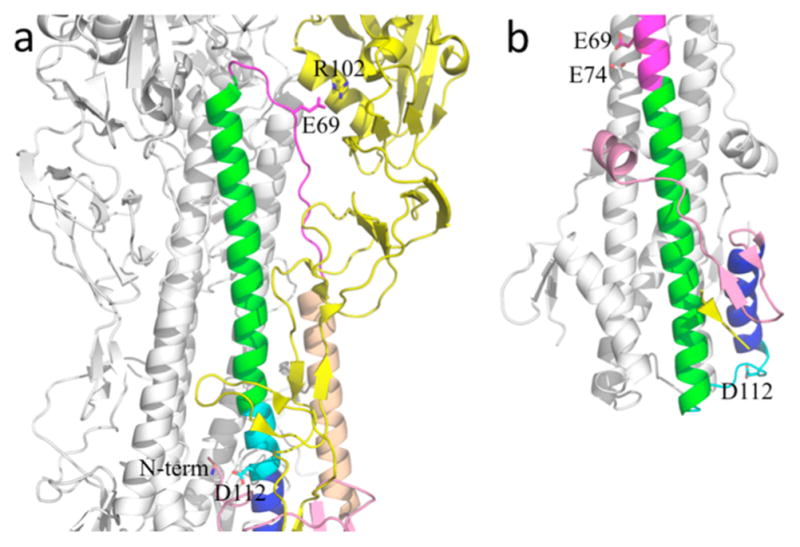
pH-dependent conformational transition of hemagglutinin. (a) In the prefusion form (Protein Data Bank entry 1JSD), HA2 Asp112 forms an ion pair with the N-terminal amino group, and HA2 Glu69 forms a salt bridge with HA1 Arg102. (b) In the postfusion form (PDB entry 1HTM), the N-terminus of the HA2 central helix extends from residue 74 to residue 37, while residues 106–113 (in cyan) become a loop, allowing the C-terminal helical segment (in blue) to fold back. HA2 Asp112 is now exposed on the surface, and Glu69 forms a pair with Glu74 of a neighboring chain.
