Abstract
Background
We recently demonstrated that the heart of late pregnant (LP) rodents is more prone to ischemia/reperfusion (I/R) injury compared to non-pregnant rodents. Lipids, particularly polyunsaturated fatty acids, have received special attention in the field of cardiovascular research. Here, we explored whether Intralipid (ITLD) protects the heart against I/R injury in LP rodents and investigated the mechanisms underlying this protection.
Methods and Results
In-vivo female LP rat hearts or ex-vivo isolated Langendorff-perfused LP mouse hearts were subjected to ischemia followed by reperfusion with PBS or ITLD (one bolus of 5mg/kg of 20% in in-vivo and 1% in ex-vivo). Myocardial infarct size, mitochondrial calcium retention capacity, genome-wide expression profiling, pharmacological inhibition and co-immunoprecipitation were performed. One bolus of ITLD at reperfusion significantly reduced the in-vivo myocardial infarct size in LP rats (23.3±2% vs 55.5±3.4% in CTRL, p<0.01). Postischemic administration of ITLD also protected the LP hearts against I/R injury ex-vivo. ITLD significantly increased the threshold for the opening of the mitochondrial permeability transition pore in response to calcium overload (nmol-calcium/mg-mitochondrial protein: 290±17 vs. 167±10 in CTRL, p<0.01) and significantly increased phosphorylation of STAT3 (1.8±0.08 vs. 1±0.16 in CTRL, p<0.05) and GSK-3β (2.63±0.55 vs. 1±0.0.34 in CTRL, p<0.05). The ITLD-induced cardioprotection was fully abolished by Stattic, a specific inhibitor of STAT3. Transcriptome analysis revealed caveolin 2 (Cav2) was significantly upregulated by ITLD in hearts of LP rats under I/R injury. Co-immunoprecipitation experiments showed that Cav2 interacts with STAT3.
Conclusions
ITLD protects the heart in late pregnancy against I/R injury by inhibiting the mPTP opening through Cav2/STAT3/GSK-3β pathway.
Keywords: Pregnancy, ischemia/reperfusion, Intralipid, Caveolin-2, STAT3, mPTP
INTRODUCTION
The prevalence of coronary artery disease during pregnancy has dramatically increased in women over the past decade due to increased maternal age and significant changes in womens’ lifestyle patterns including stress, smoking, diabetes and chronic hypertension1, 2. Ischemic heart disease during pregnancy carries a markedly worse prognosis than in non-pregnant women3, 4. We have recently successfully modeled this observation in rodents and demonstrated that the cardiac vulnerability to ischemia/reperfusion (I/R) injury considerably increases in LP rodents, leading to myocardial infarct size ~4-fold greater than in non-pregnant (NP) controls5. Identifying a novel and safe therapy for use at the time of reperfusion would offer a great clinical opportunity in the treatment of pregnant women with cardiovascular complications.
Lipids, particularly polyunsaturated fatty acids (PUFAs), have received special attention in cardiovascular research as PUFA-rich diets are associated with a decreased risk of coronary artery disease6, 7. Intralipid (ITLD) is an emulsion of fatty acids that has been widely and safely used as a source of parenteral nutrition in patients for more than 4 decades8, 9. ITLD is composed of soybean oil (20%), egg-yolk phospholipids (1.2%) and glycerol (2.2%). We have recently shown that administration of ITLD at reperfusion reduces the myocardial infarct size in male rodents subjected to I/R both in-vivo and ex-vivo10, 11.
Delaying the opening of the mitochondria permeability transition pore (mPTP) upon reperfusion has been a potential strategy to reduce myocardial injury. We have demonstrated that ITLD inhibits the opening of the mPTP and protects the male heart by recruiting the reperfusion injury salvage kinase pathway known as RISK pathway11. The activation of the Survivor Activating Factor Enhancement (SAFE) pathway, which includes the activation of the transcription factor signal transducer and activator of transcription-3 (STAT3), is recently recognized as a ‘RISK-free’ pathway that can also confer cardioprotection12–15. Interestingly, STAT3 has been implicated recently in peripartum cardiomyopathy (PPCM)16, 17, as female mice with a cardiomyocyte-specific deletion of STAT3 develop PPCM and myocardial STAT3 protein levels are reduced in PPCM patients.
Here, we explored whether ITLD, which has been shown to be well tolerated both in adults8, 9 and even in premature underweight babies18, 19, can protect the rodent heart in LP against I/R injury both in mice and rats, and examined the mechanisms involved in ITLD-induced cardioprotection in LP.
METHODS
Animals
Female late pregnant (LP) mice (C57BL/6, 2–3 months old, day 19–20 of pregnancy) and LP rats (Sprague-Dawley, 2–3 months old, day 21–22 of pregnancy) were used. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH Publication No. 85-23, revised 1996). The animal protocol was approved by the University of California Los Angeles School Of Medicine Animal Research Committee.
Left anterior descending coronary artery occlusion and measurement of infarct size
Rats were anesthetized with a mixture of ketamine (80 mg/kg i.p.) and xylazine (8 mg/kg i.p.). The rats were intubated and ventilated with a ventilator (CWE SAR-830/P). The hearts were exposed through a left thoracotomy in the fourth intercostal space. The pericardium was opened, and a 5.0 Prolene suture was tightened around the proximal left anterior descending (LAD) coronary artery. Ischemia was confirmed by ST elevation in ECG. The heart was subjected to 45 minutes of ischemia, following by 180 minutes of reperfusion, which was achieved by releasing the tension on the ligature. One single bolus of ITLD (20%, 5ml/kg body weight) or PBS (same volume as in ITLD group) was applied via the femoral vein 5 minutes prior to reperfusion.
At the end of the experiment, the LAD coronary artery was retied and 2.5 ml of 1% Evans blue dye was injected into the femoral vein to delineate the non-infarcted region of the heart. The myocardial ischemic area at risk (AAR) was identified as the region lacking blue staining. The ventricles of the hearts were sliced transversely into 2 mm thick slices. The slices were incubated in 1% triphenyltetrazolium chloride (TTC) at 37°C for 15 min to identify the non-infarcted and infarcted areas. The infarcted area was displayed as the area unstained by TTC. Infarct size was expressed as a percentage of the AAR by area.
Langendorff preparation
Mice were anesthetized via intraperitoneal injection of pentobarbital sodium (50 mg/kg). Heparin (200 IU/kg) was also injected to prevent blood coagulation. The heart was quickly removed and perfused through the aorta in a Langendorff apparatus with Krebs Henseleit bicarbonate buffer solution (KH) (mM): glucose 11.1, NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25.0, CaCl2 2 at pH 7.4 bubbled with 95% O2/5% CO2 at 37 °C. Once equilibration was achieved, the aorta was clamped for 20 min to induce global normothermic (37°C) ischemia, followed by reperfusion with KH alone (CTRL, with additional 1% ITLD, with 1% ITLD+Stattic (20 μM), or with Stattic alone (20 μM). The duration of reperfusion was 40 minutes for infarct size and heart function measurements and 10 minutes for calcium retention capacity measurements and signaling pathways analysis.
Ex vivo Heart functional measurements and Myocardial necrosis
A catheter (1.4F Millar SPR-671) connected to a pressure transducer (Power Lab, AD Instruments) was directly inserted into the left ventricle (LV) to measure left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and heart rate (HR). The left ventricular developed pressure (LVDP) was calculated as LVDP = LVSP−LVEDP and the rate pressure product (RPP) as RPP = HR×LVDP.
At the end of reperfusion, the hearts were cut into slices and myocardial necrosis was assessed by measurement of the infarct size using triphenyltetrazolium chloride (TTC) staining. The slices were fixed in 4% paraformaldehyde. The area of necrosis was quantified by Adobe Photoshop and expressed as the percentage of total ventricular (LV) area.
Ca2+-induced mitochondrial permeability transition
Preparation of isolated mitochondria
Mitochondria were prepared as previously described8. In brief, myocardial sections of ex-vivo perfused hearts (approximately 0.15–0.22 g) were placed in isolation buffer A containing (mM): 70 sucrose, 210 mannitol, 1 EDTA and 50 Tris-HCl, pH 7.4 at 4°C. The tissue was finely minced with scissors and homogenized in the same buffer A (1 ml buffer/0.1g of tissue) using Kontes and Potter-Elvehjem tissue grinders. The homogenate was centrifuged at 1,300 g for 3 minutes; the supernatant was filtered through cheesecloth and centrifuged at 10,000 g for 10 min. The mitochondrial pellet was resuspended in isolation buffer B containing (mM): 70 sucrose, 210 mannitol, 0.1 EDTA and 50 Tris-HCl, pH 7.4. Mitochondria protein concentration was measured using the Bradford assay.
Calcium Retention Capacity (CRC)
The opening of the mPTP was assessed following in-vitro Ca2+ overload as previously described8. Free Ca2+ concentration outside the mitochondria was recorded with 0.5 μM calcium green-5N (Invitrogen) using excitation and emission wavelengths set at 500 and 530 nm, respectively. Isolated mitochondria (500 μg of protein) were suspended in 2 ml buffer C (mM, 150 sucrose, 50 KCl, 2 KH2PO4, 5 succinic acid and 20 Tris/HCl, pH 7.4). Samples were pre-incubated for 90 seconds in the spectrofluorometer cell, and CaCl2 pulses (20nmol) were applied every 60 sec in the spectrofluorometer. The Ca2+ pulses induced a peak of extra-mitochondrial Ca2+ concentration that returned to near-baseline level as Ca2+ entered the mitochondrial matrix via the uptake by the Ca2+ uniporter. With increasing calcium loading, the extra-mitochondrial Ca2+ concentration started accumulating, reflecting a lower capacity for mitochondria Ca2+ uptake, which was followed by a sustained Ca2+ increase indicating a massive release of the mitochondrial Ca2+ by the opening of the mPTP. The Ca2+ retention capacity (CRC) was defined as the amount of Ca2+ required to trigger this massive Ca2+ release which was used here as an indicator of the mPTP sensitivity to Ca2+. CRC was expressed as nmol of CaCl2 per mg of mitochondrial protein.
Pharmacological Agents and antibodies
ITLD 20% was purchased from Sigma (St. Louis, MO, USA). ITLD is made up of 20% soybean oil, 1.2% egg yolk phospholipids, 2.25% glycerol and water. The major component fatty acids are linoleic (44–62%), oleic (19–30%), palmitic (7–14%), linolenic (4–11%) and stearic (1.4–5.5%). ITLD was used at 5ml/kg in the in-vivo rat model of I/R injury and 1% in ex-vivo based on our previous work9. Sattic was purchased from Sigma (St. Louis, MO, USA) and was used at 20 μM, a concentration that has been used previously in isolated Langendorff perfused hearts to explore the role of STAT3 signal transduction pathway20. The primary antibodies used were anti-GSK-3β (rabbit monoclonal), anti-phospho GSK-3β (Ser 9, rabbit monoclonal), anti-STAT3 (mouse monoclonal), anti-phospho STAT3 (Tyr705, rabbit monoclonal), Cav2 mouse IgG1 (Clone 2297, BD 610407), and Mouse monoclonal anti-Vinculin. The secondary antibodies were goat anti-rabbit Alexa 680 from Invitrogen (Carlsbad, CA, USA) and goat anti-mouse IR Dye 800 CW from Odyssey™, Li-Cor.
Immunoblot analysis
The entire ex-vivo hearts were used for making whole heart lysates, since in this model the whole heart is considered to be area at risk. Hearts were homogenized at 4°C in (mM): 150 NaCl, 50 Tris-HCl, 1 EGTA. 1 EDTA, 1 NaF, 1 PMSF, 1 Na3VO4, 1% NP-40, 0.1% SDS and 0.5% Sodium Deoxycholate (pH 7.4) supplemented with Protease and Phospatase Inhibitor cocktails (Roche, San Francisco, CA). The samples were centrifuged at 12,000 g for 10 minutes and the supernatants were collected. Protein concentration was measured and 50–100 μg of total protein was loaded on a 4–20% gradient Tris/HCl SDS polyacrylamide gel, electrotransferred to nitrocellulose paper, blocked with 5% nonfat dry milk in 20 mM of TBS with 0.1% Tween and 0.5% Triton-X100 and incubated with primary antibodies. Blots were then indirectly labeled using infrared fluorophore-conjugated secondary antibodies for 1 h at room temperature, and visualized with the Odyssey™ Imaging System (Li-Cor, Lincoln, NE). Equal loading of protein onto each lane in the gel was confirmed with Vinculin. The proteins were first normalized to their corresponding Vinculin, and then the phosphorylated proteins were normalized to their corresponding total protein levels.
Immunoprecipitation
Immunoprecipitations were performed using protein G-agarose (Roche Applied Science, ndianapolis, IN, USA). Whole hearts were lysed in IPEGAL lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 2 mM dithiothreitol, and 0.5% Igepal CA-630 plus mammalian protease inhibitor (Sigma) and phosphatase inhibitor cocktails (Upstate, Charlottesville, VA, USA). The lysate was precleared with agarose, incubated with primary antibody for 3 hours at 4°C, immunoprecipitated overnight with proteinagarose at 4°C, and then centrifuged at 13,000 g for 5 minutes. Protein-agarose pellets were washed once with lysis buffer, followed by subsequent washes with wash buffer A (50 mM Tris-HCl, pH 7.5, 500 mM NaCl, and 0.2% Igepal CA-630) and wash buffer B (10 mM Tris-HCl, pH 7.5, and 0.2% Igepal CA-630). After the final wash, the pellet was resuspended in 30 μl of 2× SDS-PAGE sample buffer. Immunoprecipitated samples were then resolved, separated by SDS-PAGE, and subjected to immunoblot analyses using specific antibodies against the proteins of interest. The co-immunoprecipitation experiments were preformed with 4 samples from each group.
Hybridization and microarray profiling
Total RNA was isolated using the RNeasy Fibrous Tissue Mini kit (Qiagen) following the manufacturer’s protocol. RNA quality and quantity were established using a 2100 Bioanalyzer (Agilent Technologies). Biotinylated complementary RNA (cRNA) was prepared and hybridized to the Rat Gene 1.0 ST array (Affymetrix) according to the standard Affymetrix processing protocol. The array was scanned in a GeneChip Scanner 3000. The computational and statistical analysis of the microarray data was carried out using R version 2.14.2 software and the Bioconductor packages21 as described recently22, 23. Following background correction, expression data were normalized with the variance stabilization and normalization algorithm24 and log2 transformed using the median polish algorithm of robust multi-array average25. The quality of the data was assessed with the affy26 and the arrayQualityMetrics27 packages. To detect differences in transcript cluster expression between conditions, a moderated linear model was applied using the limma package28. To adjust resulting P values, the false discovery rate (FDR) was controlled. Statistical significance was considered at FDR ≤ 5%. Microarray data are deposited in the Gene Expression Omnibus database under accession No. GSE77698.
Quantitative real-time RT-PCR
Reverse transcription and quantitative real-time PCR were performed. Total RNA was reverse transcribed and amplified using One Step RT qPCR MasterMix (Eurogentec) in a LS480 Lightcycler (Roche). Reactions where RNA or reverse transcriptase had been omitted were used as negative controls. PCR products were obtained using gene-specific, intron-spanning primers. The levels of all candidate genes were normalized to GADPH housekeeping mRNA levels. Cav2 primer (forward: GCTCAACTCTCATCTCAAGCT, reverse: TCTGTCACACTCTTCCATATC). GADPH primer (forward: TGCACCACCAACTGCTTAGC, reverse: GGCATGGACTGTGGTCATGAG)
Statistical Analysis
For the in vivo study, means were compared using the t-test. For the ex vivo studies, mean profiles over time were compared across groups using repeated measure analysis of variance (ANOVA) methods. When significant differences were detected, individual mean values were compared by a post hoc test, which allowed for multiple comparisons. SPSS, version 15.0, (SPSS Inc, Chicago Ill) was used to carry out the computations. As all outcomes were continuous, results were summarized with Means ± standard errors of the mean (SEM). P < 0.05 was considered statistically significant.
RESULTS
Administration of ITLD at reperfusion protects the heart in late pregnancy against in-vivo I/R injury
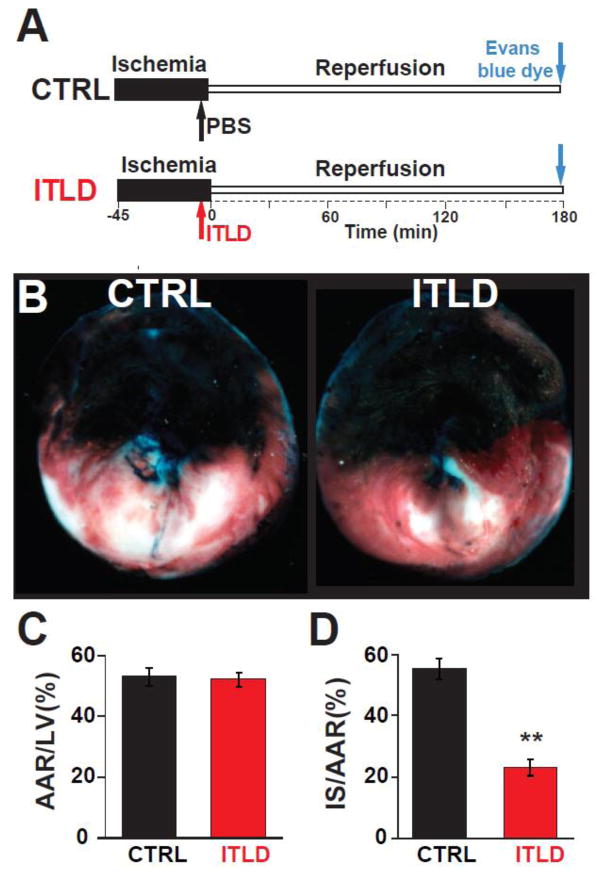
Recently, we showed that the heart is more susceptible to I/R injury in late pregnancy compared to NP, as the infarct size was ~4-fold larger in LP compared to NP rats5. Here, we examined whether administration of ITLD at reperfusion protects that heart in LP rats against I/R injury (Fig. 1A). One bolus of ITLD at reperfusion reduced the myocardial infarct size in LP rats as it can be clearly seen in the representative images of the heart sections after TTC staining in Fig 1B. The area at risk (AAR) to LV ratio was similar in both groups (53.1±2.9% in CTRL vs. 52.3±2.3% in ITLD, p>0.05, Fig 1C), indicating that the two groups were subjected to a comparable degree of ischemic risk. However, the infarct size was significantly smaller in the ITLD group compared to control; the ratio of infarct size to AAR was 23.3±2.6% in ITLD vs. 55.6±3.4% in control (p<0.01, Fig. 1D). These data demonstrate that one bolus of ITLD right before reperfusion is sufficient to protect the LP heart against I/R injury in-vivo.
Figure 1. ITLD reduces the myocardial infarct size in LP rats subjected to in-vivo I/R injury.
A. Experimental protocol, the left coronary artery was occluded for 45 minutes followed by 3 hours of reperfusion. One single IV bolus of PBS (control group, CTRL) or 20% ITLD (5ml/kg body weight, ITLD) was administered 5 min before reperfusion. B. Representative cross sections of LP hearts in CTRL and ITLD groups. C, Percentage of area at risk (AAR) divided by LV and D. infarct size (IS) divided by AAR (D) in CTRL and ITLD groups. **p<0.01 vs. CTRL (n=6–7).
Post-ischemic administration of ITLD protects isolated hearts against ex vivo I/R injury
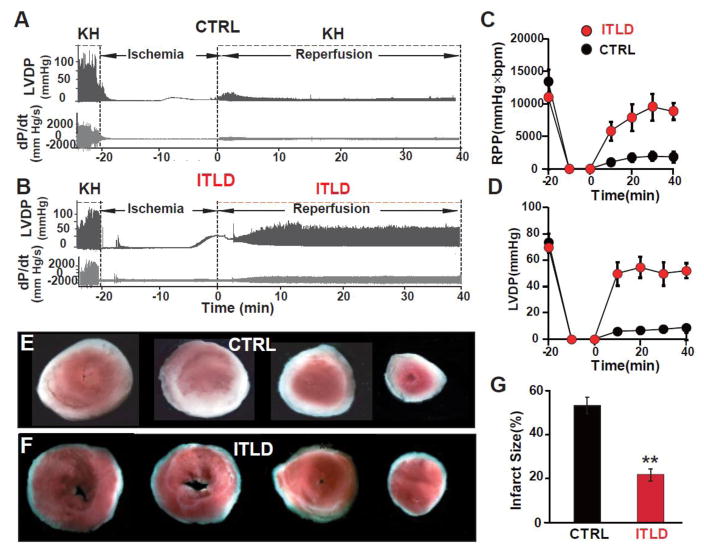
Next, we examined if ITLD could also protect the hearts of LP mice against ex vivo I/R injury. Post-ischemic administration of ITLD significantly improved the heart functional recovery in mice (RPP=8881±1331 mmHg×beats/min in ITLD vs. 1856±860 mmHg×beats/min in CTRL, p<0.01, at 40 min of reperfusion, Fig. 2C). LVDP was also significantly larger in ITLD group compared to CTRL at the end of reperfusion representing increased cardiac performance post ischemic insult (LVDP=52.0±5.5 mmHg vs. 8.8±2.8 mmHg in CTRL, p<0.01, Fig. 2D). In agreement with a better functional recovery in the ITLD group hearts, the myocardial infarct size was significantly smaller in the ITLD group compared to CTRL (21.7±2.6% vs. 53.5±3.7% in CTRL, p<0.01, Fig. 2E–G). These data suggest that ITLD protects both mice and rats from myocardial I/R injury.
Figure 2. Administration of ITLD during reperfusion improves heart functional recovery and reduces myocardial infarct size of LP mice subjected to ex-vivo I/R injury.
A, B. Representative traces of the left ventricular pressure and heart contractility as a function of time in CTRL and 1% ITLD group, respectively. C. Rate Pressure Product (RPP) and D. left ventricular developed pressure (LVDP) as a function of time in CTRL and ITLD. E, F. Four slices of the same heart after TTC staining in CTRL and ITLD, respectively. The white area represents the infarct zone and red shows the viable area. G. The area of necrosis as the percentage of total left ventricular (LV) area in CTRL and ITLD. *p<0.05 and **p< 0.01 vs. CTRL (n=7–8).
ITLD inhibits the opening of the mitochondrial permeability transition pore
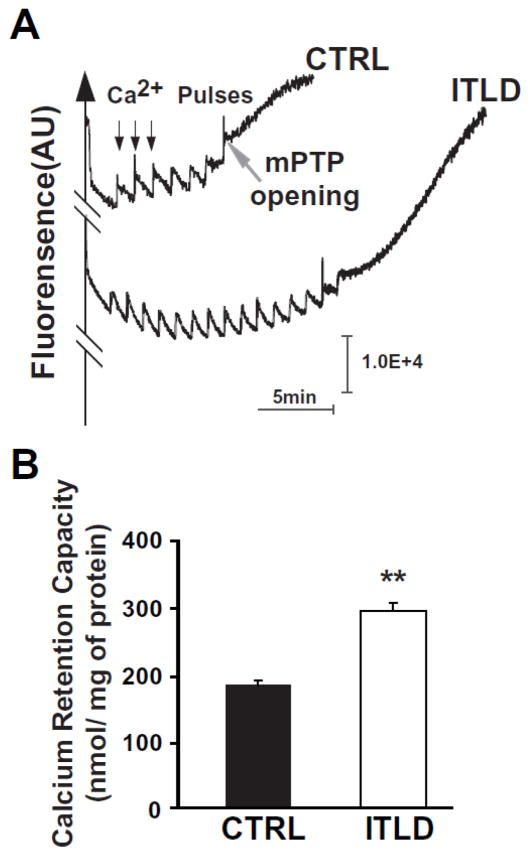
We have previously shown that the threshold for the opening of the mPTP in isolated cardiac mitochondria is significantly lower in LP compared to NP mice5. We therefore compared the threshold for the opening of the mPTP in response to calcium overload in isolated mitochondria from LP hearts reperfused with KH buffer (CTRL) or ITLD. A typical example of the time course of Ca2+ concentration in the mitochondrial external medium is shown in Fig. 3A. In this example, 7 pulses of 20 nmol/L Ca2+ were sufficient to trigger the opening of the mPTP in the CTRL group. Interestingly, the calcium load significantly increased in mitochondria isolated from ITLD group, as the number of calcium pulses required for the opening of the mPTP was increased to 15 pulses. Similar results were obtained in 5 other experiments. The calcium retention capacity (CRC) was significantly higher in the ITLD group compared to CTRL (290±18 nmol/mg mitochondrial protein in ITLD vs. 167±10 nmol/mg mitochondrial protein in CTRL, p<0.01).
Figure 3. ITLD inhibits the opening of the mPTP in LP mice subjected to I/R injury.
A. Typical recording of the mPTP opening in isolated mitochondria from CTRL and ITLD group. B. Calcium retention capacity (CRC) quantified in CTRL and ITLD. *p<0.05 vs. CTRL (n=6).
ITLD induces phosphorylation of STAT3 and GSK-3β in the heart in LP
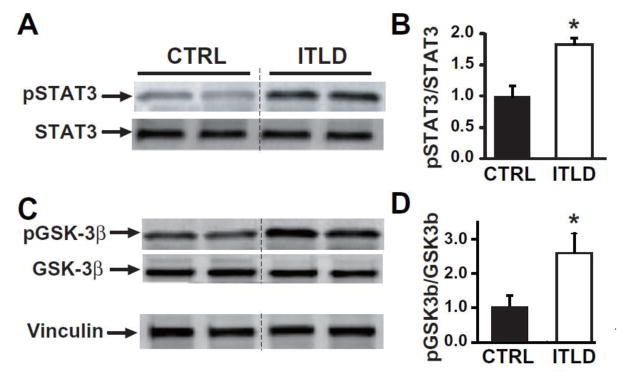
Our mechanistic investigations revealed that the ITLD-induced cardioprotection in LP was associated with a significant increase in the phosphorylation of STAT3 (in arbitrary units normalized to control: 1.81±0.08 vs. 1±0.16 in CTRL, p<0.05, Fig 4A, B) and GSK-3β (in arbitrary units normalized to control: 2.63±0.55 vs. 1±0.34 in CTRL, p<0.05, Fig. 4C, D).
Figure 4. ITLD upregulates phosphorylation of STAT3 and GSK-3β in LP mice subjected to I/R injury.
A, C. Representative immunoblots of pSTAT3, total STAT3, pGSK-3β, and total GSK-3β in CTRL and ITLD group. B, D. Western Blot quantification of pSTAT3 and total STAT3, and pGSK-3β to total GSK-3β ratios in CTRL and ITLD. *p<0.05 vs. CTRL (n=3–6).
ITLD-induced cardioprotection in LP is fully abolished in the presence of a specific inhibitor of STAT3
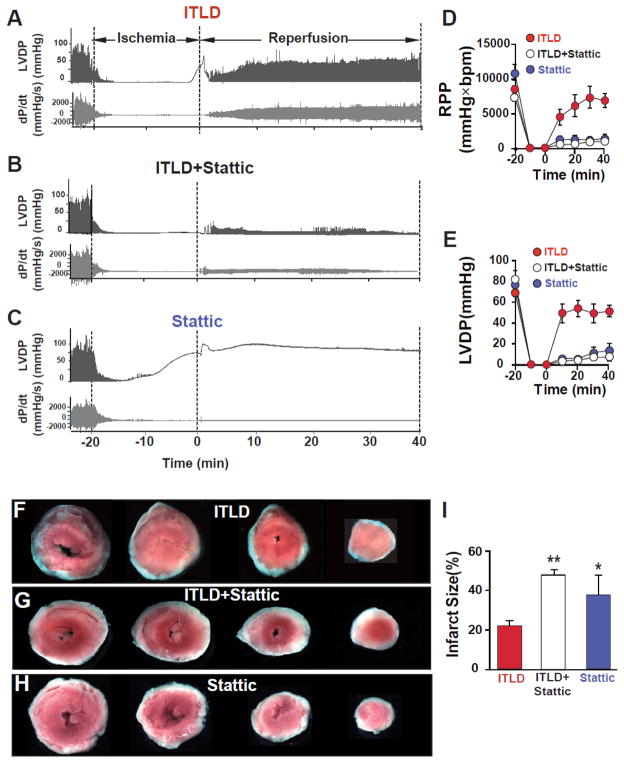
To further examine the involvement of the STAT3 pathway in ITLD-induced cardioprotection in LP, isolated mouse hearts were reperfused for 40 min with ITLD, STAT3 inhibitor Stattic, or ILTD together with Stattic. Typical examples of LVDP and dP/dt are shown in Fig. 5A–C. In the presence of Stattic, ITLD-induced cardioprotection was fully abolished, as RPP and LVDP were significantly lower at the end of 40 min reperfusion (RPP=8881±1331 mmHg*beats/min in ITLD vs. 1186±563 mmHg*beats/min in ITLD+Stattic, p<0.01; LVDP=52.0±5.5 mmHg in ITLD vs. 7.3±3.6 mmHg in ITLD+Stattic, p<0.01, Fig. 5D,E). The RPP and LVDP in Stattic alone group were not significantly different than ITLD+Stattic (RPP=1779±785 mmHg*beats/min and LVDP=13.6±7.2 mmHg, Fig. C–E). The infarct size was also significantly larger in ITLD+Stattic group when compared to ITLD alone (47.9±2.5% in ITLD+Stattic vs. 21.7±2.6% in ITLD, p<0.01, Fig. 5F,G,I). The infarct size in Stattic group was not significantly different than ITLP+Stattic (37.64±9.8, p=0.2, Fig. H,I). These data further confirm the role of the STAT3 pathway in ITLD-induced cardioprotection.
Figure 5. Involvement of STAT3 signaling pathway in ITLD-induced cardioprotection against I/R injury in LP.
A, B, C. Representative traces of the left ventricular pressure and heart contractility as a function of time in ITLD (1%), ITLD+Stattic (20 μM), and Stattic (20 μM) groups. D. Rate pressure product (RPP) and E. left ventricular developed pressure (LVDP) as a function of time in ITLD (n=7), ITLD+Stattic (n=7), and Stattic (n=4). F, G, H. Four slices of the same heart after TTC staining in ITLD, ITLD+Stattic, and Stattic groups. The white area represents the infarct zone and red shows the viable area. I. The area of necrosis as the percentage of total left ventricular (LV) area in ITLD (n=7), ITLD+Stattic (n=7), and Stattic (n=4). *p<0.05 and **p< 0.01 vs. CTRL (n=7–8).
Transcriptome analysis reveals Cav2 regulation by ITLD in hearts of LP rats under I/R
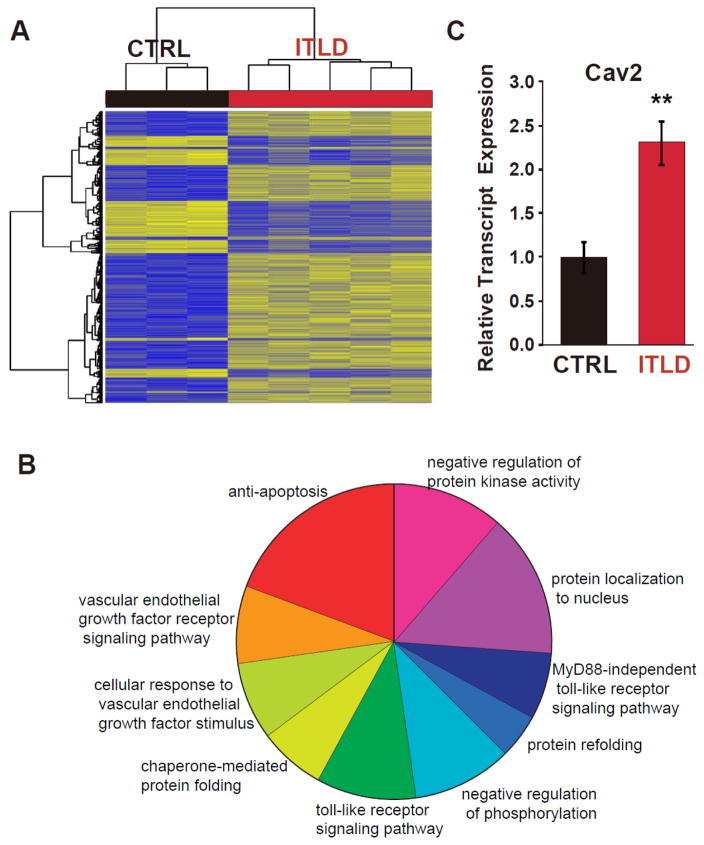
To identify novel candidates that might be involved in ITLD-induced cardioprotection against I/R injury in LP, we performed genome-wide expression profiling using the hearts of LP rats subjected to in-vivo I/R that received either a bolus of ITLD or PBS at reperfusion (Fig. 1A). Our analysis revealed that 811 transcript clusters were significantly regulated (adjusted p<0.05); among them, 160 genes were upregulated by more than 1.5-fold, whereas 20 genes were downregulated by more than 2-fold (Fig. 6A and Supplemental Table 1). Gene Ontology of differentially expressed genes show pathways involved in anti-apoptosis, vascular endothelial growth factor receptor signaling, protein localization to the nucleus, protein kinase activity, and toll-like receptor signaling are among the most significantly affected pathways in pregnancy by ITLD.
Figure 6. Genome-wide expression profiling revealed the Cav2 gene as a novel candidate regulated by ITLD in the heart of LP rats subjected to I/R injury.
A. Unsupervised hierarchical clustering of the ITLD-dependent differentially expressed transcript cluster expression in LP under I/R conditions and the intervention factor of the RNA source for CTRL and ITLD. Blue indicates low expression and yellow indicates high expression. B. Gene Ontology of differentially expressed genes show anti-apoptosis, vascular endothelial growth factor receptor signaling pathway, protein localization to nucleus, protein kinase activity, and toll-like receptor signaling pathway among the most significantly affected pathways in pregnancy by ITLD. C. The expression of Cav2 in CTRL and ILTD groups using real-time PCR.
Among the genes that were significantly regulated by ITLD in the hearts of LP rats subjected to I/R injury, we searched for a novel protein that could potentially interact with STAT3 and regulate cardiac function in pregnancy. Since it has been shown that Caveolin-2 regulates STAT3 transcriptional activation in response to insulin29, 30, we focused on Cav2 and investigated a possible interaction between Cav2 and STAT3 in the context of myocardial I/R injury in pregnancy. Cav2 was significantly upregulated (~1.6 fold) in the ITLD group compared with the CTRL group (adjusted p=0.01). Upregulation of Cav2 expression was validated by RT-PCR (normalized to CTRL group, 1±0.17 vs. 2.3±0.24 in ITLD group, p=0.0025, Fig. 6C).
ITLD promotes physical association of Cav2 and STAT3 in the heart in LP
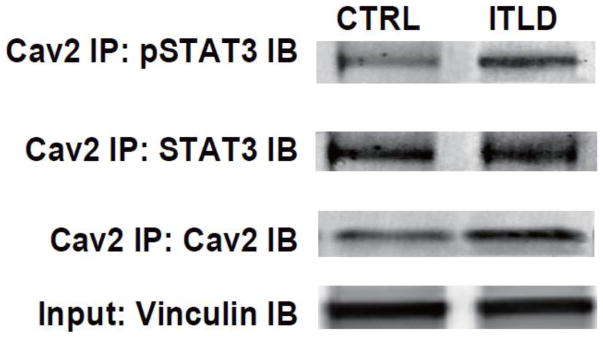
Cav2 has been shown to interact with STAT3 and is critical for phosphorylation of STAT329, 30. To examine whether ITLD promotes association of Cav2 with pSTAT3, we performed immunoprecipitation using Cav2 antibody with the heart lysates of LP subjected to I/R injury. A representative example of a co-immunoprecipitation experiment in Fig. 7 shows Cav2 interacts with STAT3 since STAT3 was detected in the heart lysates from both CTRL and ITLD groups that were immunoprecipitated with anti-Cav2. Interestingly, phosphorylation of STAT3 in heart lysates immunoprecipitated with Cav2 antibody was increased in ITLP group (Fig. 7). These data suggest that Cav2 and STAT3 are in close proximity and there is a sub-pool of STAT3 that becomes phosphorylated while bound to Cav2 in response to ITLD treatment.
Figure 7. ITLD increases phosphorylation of STAT3 interacting with Cav2.
Heart lysates from in-vivo I/R were immunoprecipitated with anti-Cav2 antibodies and subjected to immunoblot analysis with anti-Tyr705-STAT3, anti-STAT3, and anti-Cav2 antibodies as indicated. The co-immunoprecipitation experiments were preformed with 4 samples from each group (n=4) and the results were similar. A representative result from immunoprecipitation experiments with anti-STAT3 or anti-Cav2 antibodies is shown..
DISCUSSION
In the present study, we discovered that postischemic administration of ITLD, a safe lipid emulsion for human use, reduces myocardial infarct size in LP rats in-vivo. ITLD also significantly improves heart functional recovery and reduces the infarct size of isolated LP mice subjected to ex-vivo I/R injury. We also found that ITLD reduces the threshold for triggering of mPTP opening in response to Ca2+ overload. Our data highlight the role of the STAT3 pathway in ITLD-induced cardioprotection in LP rodents, since ITLD failed to protect the heart in the presence of a specific inhibitor of STAT3. Interestingly Cav2, which is critical for phosphorylation of STAT3, is significantly upregulated by ITLD treatment in the hearts of LP rats that are subjected to in-vivo I/R injury. ITLD also promotes physical association of Cav2 with STAT3. Our findings support the view that ITLD, which is safe and well tolerated, could be an ideal pharmacological agent for protecting the heart in pregnancy against I/R injury by promoting association of STAT3 and Cav2.
The mPTP is a large non-selective conductance pore located in the inner membrane of mitochondria. The mPTP remains closed during ischemia, but opens during the reperfusion period31, 32. The opening of the mPTP during reperfusion has been implicated in cell death33, 34. We have previously demonstrated that the higher susceptibility of LP hearts to I/R injury is associated with higher mPTP sensitivity to Ca2+, as the mitochondrial Ca2+ uptake required for the opening of the mPTP was significantly lower in LP hearts compared to NP5. The homeostasis of cardiomyocytes in regulating calcium overload maybe decreased in late pregnancy, resulting in a lower threshold for triggering the opening of the mPTP. Delaying the opening of the mPTP upon reperfusion has been a potential target to reduce myocardial injury35. GSK-3β phosphorylation has also emerged as an end effector, where multiple protective signaling pathways converge and regulate mPTP opening. Here, we report that ITLD-induced cardioprotection is associated with inhibition of the mPTP and increased phosphorylation of GSK-3β in the heart of LP mice.
STAT3 phosphorylation induces the rapid activation of the pro-survival kinase signaling cascades, which play an important role in reperfusion injury36. The role of STAT3 has been highlighted in cardioprotection, as ischemic postconditioning failed to protect STAT3-deficient mice37. STAT3 is also necessary for protecting LP mice against peripartum cardiomyopathy, as female mice with a cardiomyocyte-specific deletion of STAT3 develop postpartum cardiomyopathy with a very high mortality rate. In addition, STAT3 protein levels are reduced in PPCM patients16, 38. We have previously shown that the phosphorylation of STAT3 was downregulated during reperfusion in the hearts of LP mice5. Here we report that ITLD increases phosphorylation of STAT3 in LP hearts. This action of ITLD seems specific to late pregnancy, since ITLD failed to restore the downregulation of STAT3 phosphorylation in male mice subjected to I/R injury39. Our data also show that ITLD-induced cardioprotection in LP mice is fully abolished in the presence of STAT3 inhibitor, Stattic, at 20 μm. This dose of Stattic is well within the range used by previous investigators in the setting of I/R injury (10–100 μM)20, 40–42 and is also effective in disruption of STAT3 as seen in STAT3-KO mice41. Furthermore, the specificity of Stattic is not of concern since Stattic is highly specific for STAT3 inhibition, and it is previously shown not to affect other well known protective pathways in I/R injury such as ERK1/2, Akt or JAK1/243. We speculate that the STAT3 pathway is a major protective pathway activated by ITLD, since both infarct size and heart functional recovery parameters are not significantly different between LP hearts that received KH (CTRL group) or ITLD+Stattic. If cardioprotective action of ITLD in LP was mediated even partially through an alternative pathway leading to GSK-3B phosphorylation, blocking STAT3 pathway would still result in some degree of protection, but that was not the case. Therefore, we have highlighted Cav2/STAT3/GSK-3β as the major pathway mediating the cardioprotective effects of ITLD in hearts from LP animals subjected to I/R injury.
Recent studies indicate that STAT3 is also localized to the mitochondria and is important in regulation of the activity of the electron transport chain (ETC). Deletion of STAT3 in cardiomyocytes decreases the activity of complexes I and II of the ETC, leading to a decreased rate of oxidative phosphorylation44, 45. Overexpresion of mitochondria-targeted STAT3 in cardiomyocytes protects mitochondria during stress and results in lower ROS production in the mitochondria and reduced cytochrome c release during ischemia46. We postulate that during cell stress, ITLD recruits STAT3 to mitochondria which results in the inhibition of mPTP leading to cardioprotection.
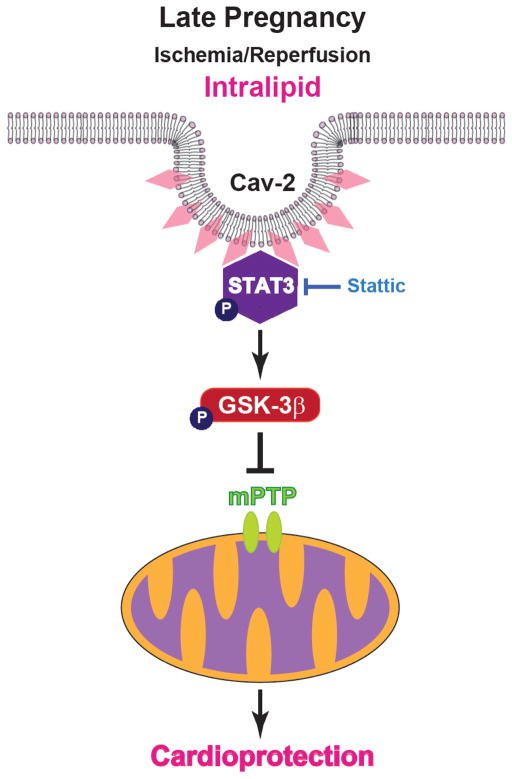
Previously, STAT3 activation has been shown to be regulated by Cav2 in response to insulin29, 30. Caveolins are critical membrane proteins of caveolae and three members are known within the caveolin protein family: Cav1, Cav2), and Cav347. Although all three Cav proteins are expressed in cardiaomyocytes48, 49, the role of Cav2 in the heart is still not known. It has been demonstrated that Cav1 and Cav3 are involved in cardiac I/R injury50, 51. However, there have been no reports demonstrating that Cav2 per se can modulate signaling in a manner similar to Cav1 and Cav3 in the heart. Our transcriptomic analysis revealed Cav2 as one of the key genes involved in ITLD-induced cardioprotection in LP under I/R, as its expression was significantly upregulated by ITLD treatment. Our data suggest that Cav2 and STAT3 are in close proximity in the heart in LP and there is a sub-pool of STAT3 that becomes phosphorylated while bound to Cav2 in response to ITLD treatment. Fig. 8 summarizes our hypothetical scheme of the mechanism of protection by ITLD against I/R injury in LP. ITLD administration at the time of reperfusion protects the heart by recruiting Cav2, which interacts with STAT3 and increases the phosphorylated levels of STAT3 and GSK-3β. These pathways converge to shift the equilibrium of GSK-3β from active form (not phosphorylated) toward the GSK-3β inactive form (phosphorylated). Once GSK-3β is phosphorylated, it inhibits the opening of the mPTP, which results in cardioprotection. Here, we found that inhibition of the STAT3 signaling pathway completely abolishes the beneficial effect of ITLD. Therefore, the Cav2/STAT3/GSK-3β pathway plays a major role in the ITLD-induced cardioprotection in pregnancy.
Figure 8. Proposed mechanisms underlying ITLD-induced cardioprotection against I/R injury in late pregnancy.
Administration of ITLD at the time of reperfusion protects the heart in LP by recruiting Cav2 resulting in increased phosphorylation of STAT3, which converges to phosphorylate GSK-3β, and, in turn, inhibits the opening of the mPTP and induces protection against reperfusion injury. The protection provided by ITLD is abolished by the STAT3 specific inhibitor, Stattic.
Given the increase in incidence of ischemic heart disease in pregnant women over the past decade, it is increasingly important to identify cardioprotective agents that are safe for both pregnant women and their babies. Recent studies demonstrate that treatment of pregnant women with beta blockers could lead to adverse pregnancy outcomes with undesirable effects on the fetus52, 53. Pregnant women treated with beta blockers are at higher risk of delivering babies with birth weights below the 10th percentile when compared to women who did not receive beta blockers during pregnancy53. Taking together, these data indicate that beta blockers are not a viable treatment option for pregnant women. ITLD is a safe emulsion of fatty acids that has been widely used as a source of parenteral nutrition in patients for more than 4 decades8, 9. Since it is known to be safe and well tolerated by premature babies, ITLD could be a safer alternative to beta blocker treatment in pregnant women18, 19.
In conclusion, ITLD, a clinically safe compound, fully reverses high vulnerability of hearts in late pregnancy subjected to I/R injury by inhibiting the mPTP opening mainly via the Cav2/STAT3/GSK-3β pathway. ITLD may serve as a novel therapeutic agent for pregnant women with cardiovascular complications, which certainly warrants further investigation.
Supplementary Material
Highlights.
Intralipid protects the heart against I/R injury in late pregnancy in rodents
Intralipid-induced cardioprotection in late pregnancy is fully abolished in the presence of a specific inhibitor of STAT3
Intralipid inhibits the opening of the mitochondrial permeability transition pore through Cav2/STAT3/GSK-3b pathway
Acknowledgments
This work was supported by R01HL131182 (ME), and AHA16GRANT27760058 (ME).
Footnotes
Disclosures:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006 Mar 28;113(12):1564–71. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 2.Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005 Mar;105(3):480–4. doi: 10.1097/01.AOG.0000151998.50852.31. [DOI] [PubMed] [Google Scholar]
- 3.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. Ann Intern Med. 1996 Nov 1;125(9):751–62. doi: 10.7326/0003-4819-125-9-199611010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Kealey A. Coronary artery disease and myocardial infarction in pregnancy: a review of epidemiology, diagnosis, and medical and surgical management. Can J Cardiol. 2010 Jun;26(6):185–9. doi: 10.1016/s0828-282x(10)70397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Umar S, Iorga A, Youn JY, Wang Y, Regitz-Zagrosek V, et al. Cardiac vulnerability to ischemia/reperfusion injury drastically increases in late pregnancy. Basic Res Cardiol. 2012 Jul;107(4):271. doi: 10.1007/s00395-012-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gylling H, Miettinen TA. A review of clinical trials in dietary interventions to decrease the incidence of coronary artery disease. Curr Control Trials Cardiovasc Med. 2001;2(3):123–8. doi: 10.1186/cvm-2-3-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt EB, Skou HA, Christensen JH, Dyerberg J. N-3 fatty acids from fish and coronary artery disease: implications for public health. Public Health Nutr. 2000 Mar;3(1):91–8. doi: 10.1017/s1368980000000112. [DOI] [PubMed] [Google Scholar]
- 8.Hansen LM, Hardie BS, Hidalgo J. Fat emulsion for intravenous administration: clinical experience with intralipid 10% Ann Surg. 1976 Jul;184(1):80–8. doi: 10.1097/00000658-197607000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calder PC, Jensen GL, Koletzko BV, Singer P, Wanten GJ. Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med. 2010 May;36(5):735–49. doi: 10.1007/s00134-009-1744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Iorga A, Sharma S, Youn JY, Partow-Navid R, Umar S, et al. Intralipid, a clinically safe compound, protects the heart against ischemia-reperfusion injury more efficiently than cyclosporine-A. Anesthesiology. 2012 Oct;117(4):836–46. doi: 10.1097/ALN.0b013e3182655e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, et al. Phosphorylation of GSK-3beta Mediates Intralipid-induced Cardioprotection against Ischemia/Reperfusion Injury. Anesthesiology. 2011 Aug;115(2):242–53. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecour S. Multiple protective pathways against reperfusion injury: a SAFE path without Aktion? J Mol Cell Cardiol. 2009 May;46(5):607–9. doi: 10.1016/j.yjmcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008 Jul 1;79(1):127–33. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 14.Pedretti S, Raddatz E. STAT3alpha interacts with nuclear GSK3beta and cytoplasmic RISK pathway and stabilizes rhythm in the anoxic-reoxygenated embryonic heart. Basic Res Cardiol. 2011 May;106(3):355–69. doi: 10.1007/s00395-011-0152-5. [DOI] [PubMed] [Google Scholar]
- 15.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008 Nov;120(2):172–85. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007 Feb 9;128(3):589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012 May 17;485(7398):333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomsits E, Pataki M, Tolgyesi A, Fekete G, Rischak K, Szollar L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr. 2010 Oct;51(4):514–21. doi: 10.1097/MPG.0b013e3181de210c. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low-birth-weight infants. J Perinatol. 2004 Aug;24(8):482–6. doi: 10.1038/sj.jp.7211114. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Gu H, Yu Q, Manukyan MC, Poynter JA, Wang M. Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS One. 2011;6(12):e29246. doi: 10.1371/journal.pone.0029246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014 Nov;16(11):1160–7. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 23.Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, et al. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol. 2012 Jan 24;59(4):410–7. doi: 10.1016/j.jacc.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Huber W, von HA, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96–104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- 25.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003 Apr;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004 Feb 12;20(3):307–15. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 27.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics--a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009 Feb 1;25(3):415–6. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004 Dec 10;306(5703):1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 29.Jeong K, Kwon H, Lee J, Jang D, Pak Y. Insulin-response epigenetic activation of Egr-1 and JunB genes at the nuclear periphery by A-type lamin-associated pY19-Caveolin-2 in the inner nuclear membrane. Nucleic Acids Res. 2015 Mar 31;43(6):3114–27. doi: 10.1093/nar/gkv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon H, Jeong K, Hwang EM, Park JY, Hong SG, Choi WS, et al. Caveolin-2 regulation of STAT3 transcriptional activation in response to insulin. Biochim Biophys Acta. 2009 Jul;1793(7):1325–33. doi: 10.1016/j.bbamcr.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998 Aug 10;1366(1–2):79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, et al. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002 Oct;4(5):769–81. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 33.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 Jul 15;341(Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- 34.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995 Jul 17;1241(2):139–76. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 35.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003 Dec 1;60(3):617–25. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 37.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009 Nov 1;84(2):201–8. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 38.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012 May 17;485(7398):333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lou PH, Lucchinetti E, Zhang L, Affolter A, Schaub MC, Gandhi M, et al. The mechanism of Intralipid(R)-mediated cardioprotection complex IV inhibition by the active metabolite, palmitoylcarnitine, generates reactive oxygen species and activates reperfusion injury salvage kinases. PLoS One. 2014;9(1):e87205. doi: 10.1371/journal.pone.0087205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L, Tsun J, Sun L, Kwan J, Watson A, Macdonald PS, et al. Critical role of the STAT3 pathway in the cardioprotective efficacy of zoniporide in a model of myocardial preservation - the rat isolated working heart. Br J Pharmacol. 2011 Feb;162(3):633–47. doi: 10.1111/j.1476-5381.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1496–H1505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manukyan MC, Alvernaz CH, Poynter JA, Wang Y, Brewster BD, Weil BR, et al. Interleukin-10 protects the ischemic heart from reperfusion injury via the STAT3 pathway. Surgery. 2011 Aug;150(2):231–9. doi: 10.1016/j.surg.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006 Nov;13(11):1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009 Feb 6;323(5915):793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010 Nov;105(6):771–85. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011 Aug 26;286(34):29610–20. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36(8):584–95. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 48.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):131–5. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 50.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007 May;21(7):1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 51.Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;(186):167–84. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 52.Babin JP, Carre M, Martin C. Possible embryofetopathy caused by beta-blocker (sotalol) taken throughout the pregnancy. Pediatrie. 1985 Mar;40(2):129–36. [PubMed] [Google Scholar]
- 53.Ersboll AS, Hedegaard M, Sondergaard L, Ersboll M, Johansen M. Treatment with oral beta-blockers during pregnancy complicated by maternal heart disease increases the risk of fetal growth restriction. BJOG. 2014 Apr;121(5):618–26. doi: 10.1111/1471-0528.12522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.