Fig. (1).
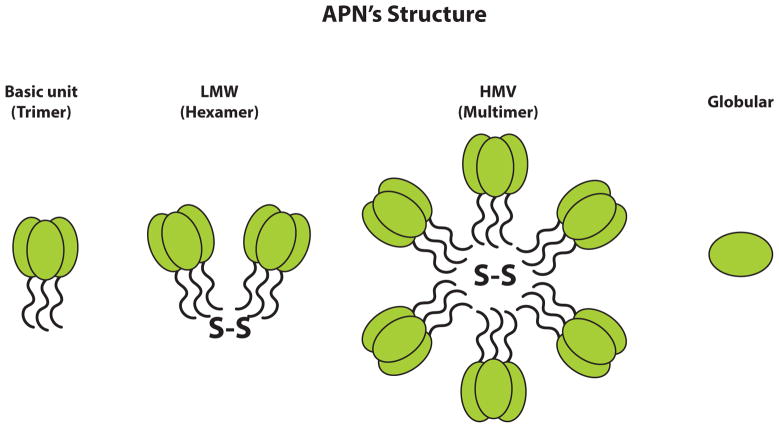
Adiponectins (APNs) exists in different isoforms, as depicted schematically, and each full-length adiponectin (composed of 244 amino acids) has a N-terminal collagen-like fibrous domain as well as a C1q-like globular domain at the C-terminus. Three adiponectin monomers combine to provide a trimeric isoform that is considered to be the basic building block for higher oligomers. Disulfide bonding allows generation of low molecular weight (LMW) and high molecular weight (HMW) complexes. A smaller adiponectin form, consisting of the globular domain alone, exists in plasma in small amounts.