Fig 3. VACV K1, C7 and C7 homologs from diverse poxviruses could bind mSAMD9L, but the C7 homolog from sheeppox virus has a specific defect due to the variation of two amino acids.
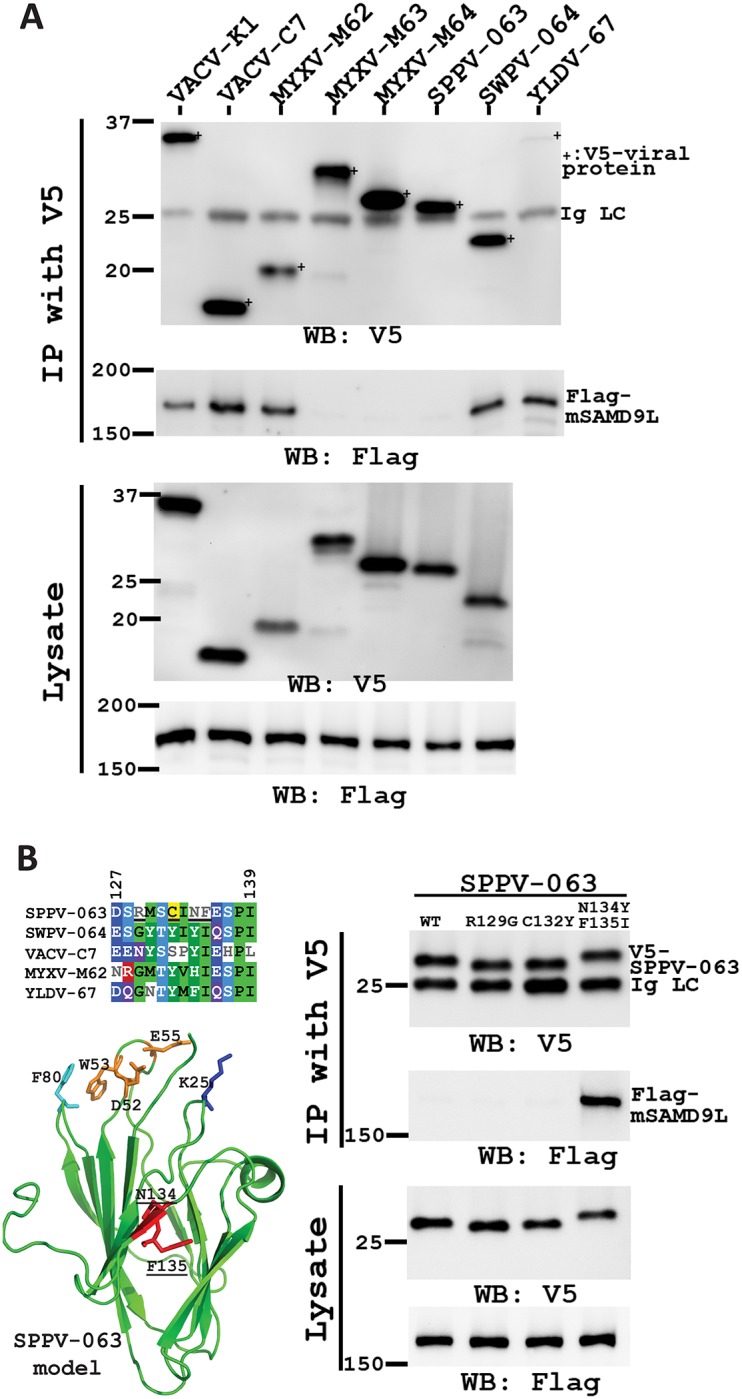
(A). The sheeppox virus C7 homolog (SPPV-063) was defective of binding mSAMD9L. 293FT cells were transfected with a plasmid expressing flag-tagged mSAMD9L and infected with the panel of vK1L-C7L--derived VACVs that expressed V5-tagged VACV-K1 or a C7 homolog from different poxviruses. The cell lysates were subjected to immunoprecipitation with an anti-V5 antibody. mSAMD9L and the epitope-tagged viral protein in the cell lysate and precipitate were detected respectively with anti-flag and anti-V5 antibody in Western blot. The light chain of the precipitated antibody (Ig LC) serves as a loading control. Due to space limitation, lysate of YLDV-67 sample was not loaded on the same gel. (B). Substitution of residue 134 and 135 of SPPV-063 restored binding to mSAMD9L. WT SPPV-063 and mutants with substitution at residue 129 (R129G), 132 (C132Y), or both 134 and 135 (N134Y, F135I) were precipitated as described in A. A multiple sequence alignment of different C7 homologues encompassing the substituted region is shown. Residues with similar physicochemical properties among at least 4 of the homologs are highlighted and colored by their properties (hydrophobic, green; negative, bright blue; positive, red; polar, purple; cysteine, yellow; alcohol, light blue). A model of SPPV-063 based on the VACV-C7 structure is also shown with critical SAMD9-binding residues labeled.
