FIG. 1.
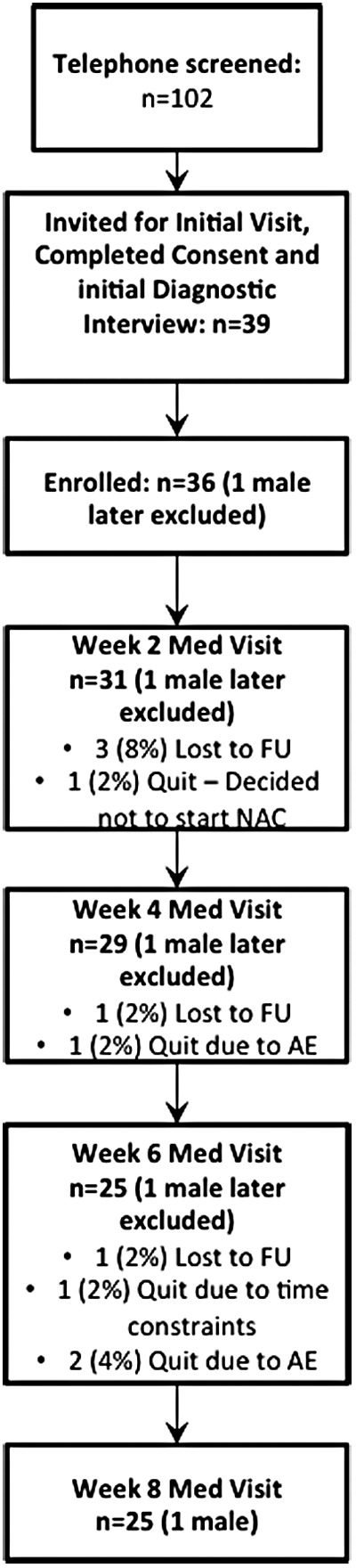
Consort diagram showing the flow of screening, recruitment, consent, enrollment, and completion of study visits for participants in this study. Thirty-six participants (one male) were enrolled and 25 (one male) participants completed the protocol. Data from the male participant were analyzed. AE, adverse events, FU, follow-up.
