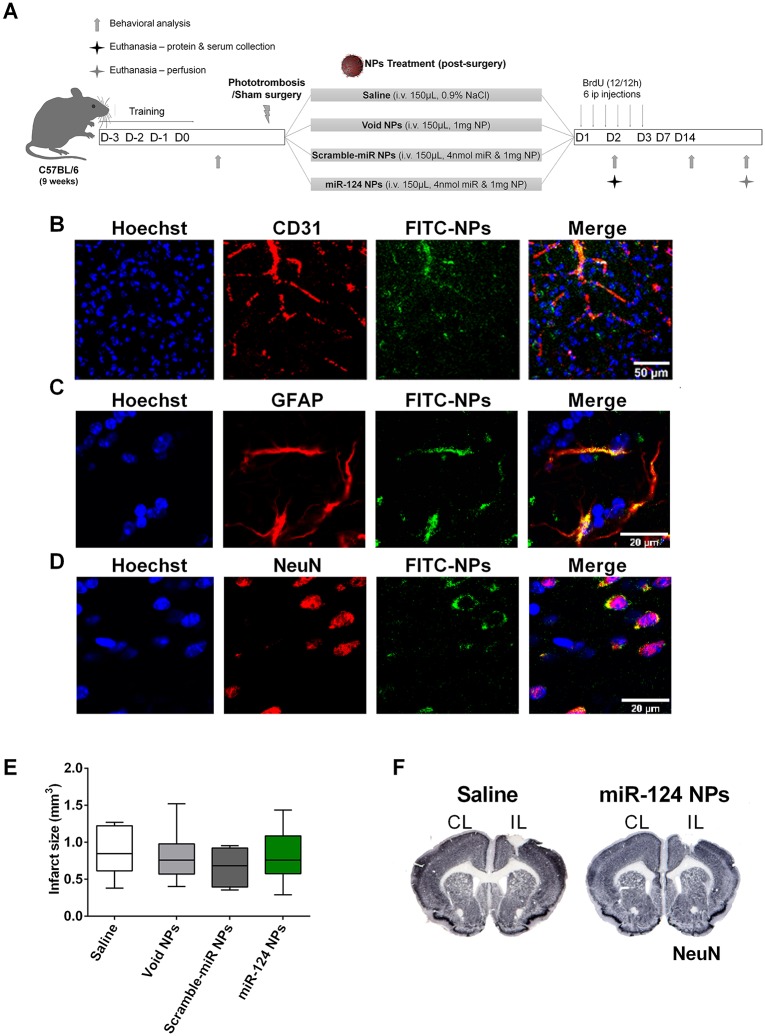
Fig 3. miR-124 NPs do not affect infarct volume of PT mice.
(A) Experimental design for the in vivo experiments. Before entering the study, mice were assigned to surgery and treatment groups subjecting them either to sham surgery or PT. The PT group was then subdivided into 4 subgroups in which mice were treated with an intravenous injection in the tail vein of saline, void NPs, scramble-miR NPs or miR-124 NPs (total of 5 different groups) immediately after surgery. Pro-inflammatory cytokines have been measured on day 2 after surgery from the ischemic core and adjacent peri-infarct tissue and serum. Infarct volumes and neurogenesis have been studied 14 days after surgery. These mice received BrdU injections (every 12 h) during the first 3 days after surgery. After a training period, neurological function was tested on days -1, 2, 7 and 14, respectively. (B, C, D) Representative photomicrographs of the peri-infarct area 4 h after intravenous injection of 1 mg of FITC-NPs (green) and stained against the nuclear marker Hoechst-33342 (blue) and either the endothelial marker CD31 (red) (B), or the astrocyte marker GFAP (red) (C), or the neuronal marker NeuN (red) (D). Scale bar 50 μm. (E) Infarct volume (in mm3) in the 4 different groups following PT. Data are expressed as medians with the 1st and 3rd quartile with the following number of animals included in each experimental group: PT saline n = 6, PT void NPs n = 6, PT scramble-miR NPs n = 8, PT miR-124 NPs n = 8. F) Representative images of the infarct area (white) in coronal sections stained with NeuN of mice treated with saline (left) or miR-124 NPs (right). Abbreviations: BrdU, 5-bromo-2'-deoxyuridine; CL, contralateral hemisphere; GFAP, glial fibrillary acidic protein; IL, ischemic hemisphere; NeuN, neuronal nuclei; NPs, nanoparticles; PT, photothrombosis.