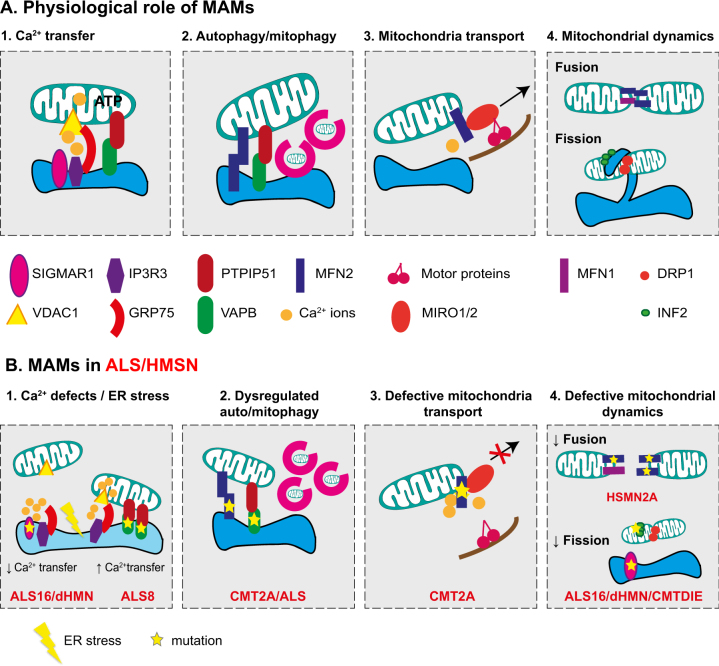
Fig. 2. Overview of the physiological roles of MAMs and potential consequences of ALS and HMSN mutations on MAM function.
a Summary of MAM proteins and their role in the maintenance of long-projecting neuron physiology. Boxes 1, 2, 3, and 4 describe the key functions handled at MAMs. (1) Calcium transfer between ER and mitochondria is mainly controlled by the IP3R3–GRP75–VDAC1 complex. Through its interaction with IP3R3, SIGMAR1 prevents IP3R3 degradation, thus maintaining calcium flux. (2) MAMs have been recently considered as a site of autophagosome formation. VAPB–PTPIP51 as well as MFN2 have been shown to regulate this process at MAMs. (3) Mitochondrial transport can be controlled at MAMs by the means of interactions between MFN2 and the MIRO1/2 proteins. MIRO proteins connect to the motor proteins kinesin and dynein in a calcium-dependent manner. (4) MAMs appear as a site-promoting fusion/fission. These processes are mainly controlled by MFN2, MFN1, DRP1, or INF2. Several proteins involved in mitochondrial dynamics such as MFN2, DRP1, or INF2 are located at MAMs. The association of DRP1, INF2, and ER tubules create a constriction ring around mitochondria favoring its fission. b Potential consequences of ALS and HMSN mutations in MAM proteins on function of long-projections neurons. Mutations in genes encoding the MAM proteins SIGMAR1, VAPB, and MFN2 have been identified in ALS or HMSN patients. This figure illustrates defects that could occur in long-projection neurons as a consequence of alteration in MAM functions. (1) Mutations in SIGMAR1 lead to loss of interactions between IP3R3 and VDAC1. Consequently, calcium transfer towards mitochondria is impaired leading to an increase of cytosolic calcium. On the contrary, mutations in VAPB reinforce its connection with PTPIP51, increasing mitochondrial calcium content as well as cytosolic calcium. In the context of both mutated SIGMAR1 and VAPB, deregulation in calcium levels impairs ER function causing ER stress. (2) Impairment of ER–mitochondria connections related to mutated MFN2 or VAPB could either promote or decrease autophagosome formation. In motoneurons differentiated from iPS cells derived from CMT2A-R94Q patients autophagic flux is increased. Whether mutated VAPB could enhance or decrease autophagy is unknown. (3) Deregulation of calcium levels due to MAM disconnection detaches MIRO proteins from the kinesin/dynein motor proteins. Consequently, mitochondrial transport is impaired. (4) Mutations in MFN2 make MFN2–MFN2 interactions non-functional leading to a reduction in fusion process. In ALS16 or distal HMN patients, loss of contacts between ER and mitochondria in neurons could prevent ER to physically enroll the mitochondria to promote fission. Mutations in INF2 can impair the step of actin polymerization required for mitochondria constriction.