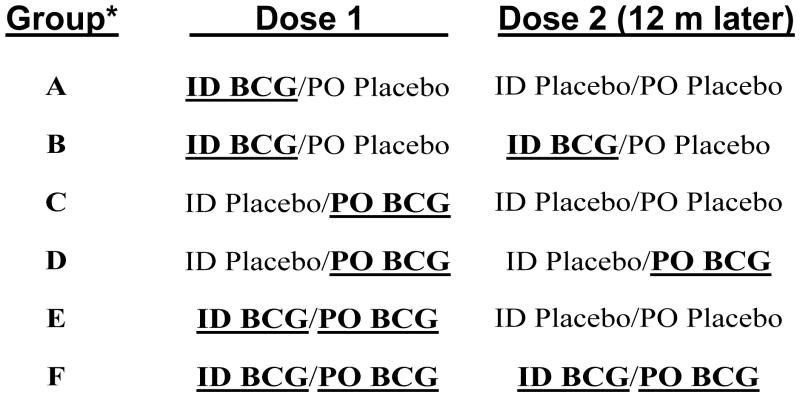
Figure 1. Overview schematic of the clinical trial.
Ten subjects were recruited into each of the groups A–F, randomized in a double blind fashion to receive PO vs ID vs PO+ID Danish BCG. An additional 8 subjects were recruited into an open label, ID Connaught BCG vaccination cohort (to compare ID immunity induced by Danish vs Connaught BCG). During year 1 of the trial, groups A and B were identical (given only ID BCG), groups C and D were identical (given only PO BCG), and groups E and F were identical (given both ID and PO BCG). Blood, tear, nasal wash and stool samples were collected at 9 different time points (pre-vaccination, and 1 week, 2 months, 6 months, 12 months, 12 months plus 1 week, 14 months, 18 months and 24 months post-vaccination).
*10 volunteers/group