Abstract
Our society faces a major challenge concerning management of the health and socio-economic burden caused by acute physical stress in the older population (+ 75 years). In particular, hip-fracture surgery (HFS) represents a major health care preoccupation, affecting 1.6 million patients worldwide, resulting in a significant drop in life quality and autonomy. The trauma is associated with 20–30% one-year mortality in the elderly. In the present study, we aim to identify factors, which influence and/or predict the outcome of elderly hip- fracture patients (HFP) post-surgery.
Our objective was to identify biomarkers with a prognostic capacity of one-year mortality. We employed an observational cohort of HFP (n = 60) followed-up longitudinally during the first year post fracture. Clinical and biological data (n = 136), collected at arrival to hospital, were then compared to healthy controls (n = 42) and analyzed using a regularized logistic regression model with lasso penalty followed by 10-fold cross-validation of variables.
We show that plasmatic neopterin levels, a molecule released by IFN-γ-activated macrophages, is predictive of mortality in HFP (ROC-AUC = 0.859). Moreover, neopterin measured at arrival to the hospital correlated negatively with the time of survival after HFS.
Neopterin therefore represents a biomarker, which enables better follow-up of patients at risk of early death.
Abbreviations: HF, hip-fracture; HFS, hip-fracture surgery; HFPs, hip-fracture patients; PBMCs, peripheral blood mononuclear cells
Keywords: Hip-fracture, Inflammation, Clinical outcome, Biomarker, Aging
Highlights
-
•
Neopterin level, measured at arrival to hospital, is a robust predictive marker of one-year mortality in HFPs.
-
•
Neopterin concentration correlated negatively with the time of survival after hip fracture surgery.
The growing incidence of hip fractures, due to demographically aging populations, represent an important burden for health care systems and for injured patients in terms of hospitalization, rehabilitation, needs for long-term care, change in autonomy and mortality. Hip fractures are associated with high rates of adverse outcome, but previous studies have not discovered methods to identify patients at high risk of pernicious clinical outcome or death. Here, we show that innate immune activation post hip fracture in older adults is associated with pernicious clinical outcome.
1. Introduction
Aging is characterized by a progressive decline of physical and mental performances. This geriatric syndrome, coined frailty, is increasing incrementally with advancing age, and more rapidly in older women and among those of lower socio-economic status. Frail older adults are at high risk of major adverse health outcomes, including disability, falls, institutionalization, hospitalization, and mortality. One of the complications of frail elderly is hip-fracture (HF).
HF is a common condition associated with a 20–30% one-year mortality in the elderly (Roche et al., 2005, Jennings and De Boer, 1999). Indeed, HF patients belong to a vulnerable group of old people with comorbid diseases and a high risk of postoperative morbidity and mortality. The high incidence of HF in elderly (3%) raises an increased concern in a world with an aging population. Thus, the number of annual HFs worldwide is actually 1.6 million, but is expected to reach > 4 million in 2050 (Gullberg et al., 1997), and has an estimated annual cost > $8 billion/year for inpatients only (Ray et al., 1997). This may reach $14 billion/year in US if including the costs that can be incurred by rehabilitation/rehospitalization over a period of one year after HFS. Consequently, a major challenge is the management of the health and socio-economic burden caused by this acute physical stress in the older population (+ 75 years). According to Bouchon's definition (Bouchon, 1984), HF represents an acute event that can participate to the accelerated decline of health in the elderly.
Factors associated with the high morbidity burden post HFS commonly include older age and cardiorespiratory comorbidities as shown in the ESCORTE study analyzing the outcomes after HFS in a large cohort of French patients (Rosencher et al., 2005). Other clinical and biological factors have been associated with mortality (Laulund et al., 2012), including gender, comorbidities (Roche et al., 2005), post-operative complications (Beloosesky et al., 2007), low albumin (Pimlott et al., 2011), high creatinine (Seyedi et al., 2015), increased post-operative troponin (Chong et al., 2009), elevated procalcitonin post-surgery (Vallet et al., 2017). Moreover, hip-fracture is known to be associated with major elevation of inflammatory cytokines, such as IL-6 or TNF-α (Briza et al., 2002, Sedlar et al., 2010, Sedlar et al., 2008, Sun et al., 2011), suggesting that inflammation may play a major role in the patients' outcome.
Neopterin (1′, 2′, 3′-d-erythro-trihydroxypropylpterin) belongs to the group of pteridines and is reported to be associated with activation of cell-mediated immunity, which is tightly associated with hyper-inflammation. Neopterin is derived in vivo from guanosine triphosphate (GTP) through a reaction catalyzed by the enzyme GTP-cyclohydrolase-I (GCH I), which is expressed by activated monocytes, macrophages, dendritic cells and endothelial cells upon stimulation by IFN-γ (Fuchs et al., 1993, Murr et al., 2002).
Here we assess how the immune system of hip-fracture patients (HFPs) copes with acute stress. Indeed, we hypothesized that biological factors associated with host immunity may predict successful recovery in older patients, defined by resilience and long-term survival after surgery. We found that increased neopterin plasmatic levels pre-surgery are predictive of non-survival post hip-fracture and may even predict time of survival after HFS. Therefore, elevated neopterin levels reflect an elevated and seemingly unregulated immune activation taking place in acute HFPs. In conclusion, neopterin measures constitute a prognostic biomarker for HFPs, which may aid to optimize patient's management and reduce public health costs.
2. Materials and Methods
2.1. Study Subjects
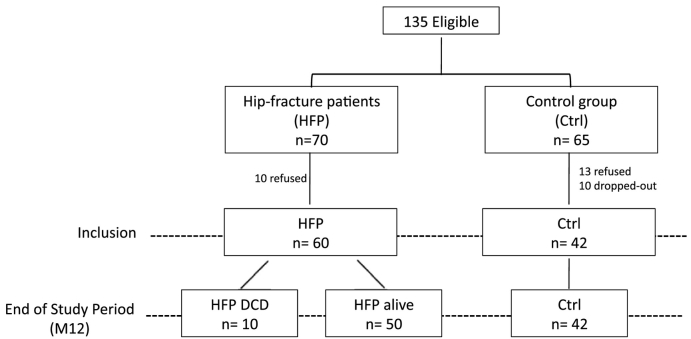
Participants were selected according to rigorous inclusion criteria: they were aged 75 years and older, free of medication and diseases affecting the immune system (e.g., cancers, autoimmune disorders), absence of prior physical disabilities and, absence of cognitive disorders. A total of 102 elderly were included for this case-control design study with half being healthy controls (n = 42) and another half suffering from hip-fracture (HF; n = 60; Fig. 1). This last group was admitted to the Department of Emergency and orthopedic surgery at Pitié-Salpetrière Hospital (Paris, France) for a fracture of the hip. Follow-up was performed up to one –year post surgery.
Fig. 1.
Design of the study.
Heparinized blood samples collected from HF patients before surgery were set as the reference point (day of arrival to hospital). For each individual, PBMCs (isolated by density gradient centrifugation) and plasma were cryopreserved until use. Experiments were performed without knowing clinical outcome which was defined at later timepoint.
2.2. Power Analysis
The required HFP cohort size was estimated from a power analysis for a biomarker with a high Cohen's d effect size of 0.8, type-1 error (α = 0.05) and type-2 error (β = 0.2) given that 1/3 of the cohort would finally display the clinical outcome, death. The latter value was obtained from literature (Roche et al., 2005, Jennings and De Boer, 1999). The HFP cohort size under these conditions is 60. Power analysis was performed using the software package G*Power (http://www.gpower.hhu.de/en.html).
2.3. Data Collection
Data collected prospectively included age, sex, previous medical history, fracture type, surgical treatment, and duration of surgery (Boddaert et al., 2014a). Associated comorbidities were carefully reviewed, and their severity was assessed using the Cumulative Illness Rating scale (CIRS), a validated scale for elderly population, in which concurring medical conditions are weighted from 0 to 4 in 13 main systems (Linn et al., 1968). According to Zekry and colleagues, the CIRS improved hospital discharge planning for elderly patients with acute disease (Zekry et al., 2012). Functional status was assessed by the Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scales. C-reactive protein (CRP) was measured by automatic laser nephelometry (BN 100 analyzer; Boehring Dade, Marburg, Germany), normal values were < 5 mg/L, and the coefficient of variation of the measurement was < 5%. Pre- and post-operative hemoglobin level, postoperative serum creatinine, estimated creatinine clearance using the Cockroft formula, and serum albumin level were recorded. Chronic renal failure was defined as an estimated Cockcroft creatinine clearance ≤ 60 mL/min, malnutrition was defined as an albumin value ≤ 35 g/L and procalcitonin concentrations were analyzed using a sandwich immunoassay based on time resolved amplified cryptate emission (TRACE) measurement (Kryptor analyzer; B.R.A.H.M.S. Thermofisher, Hennigsdorf, Germany), as previously reported (Boddaert et al., 2014a, Vallet et al., 2017).
2.4. Ethics Statement
Clinical investigation has been conducted according to Declaration of Helsinki principles. All participants were recruited in Pitié Salpétrière Hospital (Paris) and provided written informed consent. The study was approved by the “Comité de Protection des Personnes” of the Pitié Salpétrière Hospital, Paris.
2.5. Flow Cytometry Analysis
From fresh blood, absolute counts (cells per microliter) were determined using CYTO-STAT tetraCHROME kits on a FC500 cytometer (Beckman Coulter) and analyzed with Flow-Count Single Platform Method (Beckman Coulter). Directly conjugated and unconjugated antibodies were obtained from the following vendors: BD Biosciences (San Jose, CA): CD4 (HV500), CD8 (APC-Cy7), CD16 (APC-H7), CD56 (PE-Cy7); Beckman Coulter (Pasadena, CA): CD3 (ECD), CD45 (KO), NKG2A (APC), CD45RA (ECD); BioLegend (San Diego, CA): CD57 (PB), CD3 (BV650); CD8 (BV650), CD27 (AF700); R&D systems (Abingdon, UK): NKG2C (PE). Staining for cell surface markers was performed with standard method as previously described (Bayard et al., 2016). Cells were analyzed on a Fortessa flow cytometer (Becton Dickinson). Data were analyzed using FlowJo v8.2 (Tree Star, Inc) and DIVA softwares (BD Biosciences). Exhaustive phenotypic analysis of NK cells was conducted with the “FunkyCells ToolBox” software (www.FunkyCells.com).
2.6. Plasmatic Measurements
Levels of soluble CD14s (sCD14) and neopterin were determined by commercially available Elisa kits (Quantikine R&D systems and alpha diagnostic, respectively). Measures of the soluble factors IFN-inducible protein 10 (IP-10), and IL-6 were performed with the use of multiplex bead immunoassays (Biosource) and Luminex instrument. Ultrasensitive detections of IL-1β, IFN-α were obtained with Simoa technology (Quanterix). All experiments were performed by following manufacturer's instructions.
2.7. Objectives and Endpoint
Our main objective was to identify biomarkers with a prognostic capacity of one-year mortality.
2.8. Survival Score
102 patients samples were analyzed for 33 biological, 24 clinical and 79 immunological variables. These independent variables were used to predict the clinical end-point of interest (here death) in a logistic regression model. The sparse generalized linear model (GLM) was fitted by computing lasso-penalized regularization. Selected variables derived from the sparse model were validated by 10-fold cross-validation. The sparse GLM fitting was repeated (n = 500) and the most frequently identified variables were selected. To visualize the predictive power of our logistic regression model with selected independent variables we performed a partial least square discriminant analysis (PLS-DA) and plotted the first two descriptive components for each data point stratified according to their clinical status (survival). A non-normalized version of the first PLS component (PLS-C1) was finally proposed as a survival score. To quantify the accuracy of our predictive model we plotted receiving operator characteristic (ROC) curves, which depicts the specificity and sensitivity of each predicted value of a given model, and computed the area under the ROC curve (AUC). An AUC estimate approaching 1 is the result of a prediction model with maximal accuracy, whereas an AUC equal to 0.5 represents a predictive model with no predictive capacity. All statistical analysis was performed with R (v3.1.3) and the R packages: glmnet (v2.0) (Friedman et al., 2010), mixOmics (v5.0)(Le Cao et al., 2009) and pROC R package (v1.8)(Robin et al., 2011).
2.9. Statistics
Univariate statistical analysis was performed using GraphPad prism software. Groups were compared using the non-parametric Kruskal-Wallis or Mann-Whitney tests. Contingency analysis (odds ratios and Fisher's exact test) was applied for categorical variables. The linear relationship between continuous variables was analyzed with linear regression, and the corresponding Pearson's correlation coefficient (r) value was calculated. Differences in survival were analyzed with the Kaplan-Meier method. Data on surviving or non-surviving patients were censored at one-year post arrival to hospital and the results were compared with the use of log-rank test. P values < 0.05 were considered statistically significant.
3. Results
More than one hundred elderly individuals (> 75 years old) were enrolled in our study (Table 1). Approximately half of the cohort was constituted of healthy individuals (n = 42) whereas the other half was admitted in emergency department for hip-fracture (n = 60) (Fig. 1). Longitudinal follow-up post fracture was designed with blood samples taken at arrival to hospital (D0) and clinical assessment performed during hospital stay (D1-D10) and at one year post-fracture (M12). At M12, we stratified patients based on mortality (50 survivors and 10 non-survivors; Fig. 1). Clinically, we could observe that the non-survivors tend to have a slightly worth clinical state in particular regarding post-surgery complications (Table 1).
Table 1.
Patients description.
| Control group |
Hip fracture group |
p value |
||
|---|---|---|---|---|
| Survivors |
Non-survivors |
Survivors vs non-survivors | ||
| (n = 42) | (n = 50) | (n = 10) | ||
| Age (years) | 84 (77.8–99) | 87.8 (76.2–105.3) | 87.5 (78.1–96.5) | 0.89 |
| Gender (% of male) | 39 | 20 | 50 | 0.11 |
| Weight (kg) | 63 (40–95) | 55 (40–93) | 62 (39–92.5) | 0.82 |
| Vit D level (ng/mL) | 23 (7–61) | 22 (0–47) | 24 (7–62) | 0.44 |
| Albumin (g/L) | 36.9 (22–45) | 27 (19–39) | 24 (21 − 33) | 0.07 |
| CRP (g/L) | 5 (0–296) | 101 (0 − 330) | 149 (18–280) | 0.9 |
| Procalcitonin (μg/L) | nt | 0.255 (0.07–5.02) | 0.14 (0.12–0.99) | 0.94 |
| Hemoglobin (g/dL) | nt | 12.65 (8.1–15.6) | 12.3 (7.1–13.6) | 0.34 |
| Creatinine (μmol/L) | 84 (45–141) | 67 (36–157) | 107 (24–200) | 0.38 |
| Creatinine clearance (Cockroft; mL/min) | 44 (18–122) | 49.5 (15–133) | 39 (24–126) | 0.22 |
| Dindo-Clavien score | na | 2 (1–4) | 2 (1–5) | 0.01 |
| ASA score | na | 2 (1–4) | 3 (2–4) | 0.32 |
| Delay to surgery | na | 24 (3–43) | 36 (17–54) | 0.08 |
| Transfusion (%) | na | 48 | 40 | 0.74 |
| Nb of red pack cells | na | 1 (0–6) | 0 (0–5) | 0.62 |
| ADL score | 5.5 (0–6) | 5.5 (0.5–6) | 3.5 (2–6) | 0.27 |
| IADL score | 2 (0–4) | 2 (0–4) | 1 (0–4) | 0.42 |
| CIRS score | 10 (0–19) | 8 (2 − 21) | 12 (5–26) | 0.24 |
| Rockwood score | 5 (2–7) | 5 (1–7) | 5 (3–6) | 0.43 |
| Nb of medication | 6 (0 − 13) | 9 (4–15) | 9 (7–16) | 0.22 |
| Prior institution (%) | 14.6 | 20 | 0 | 0.19 |
| Ability to walk (%) | 95.2 | 87.5 | 100 | 0.58 |
| independent | 60 | 50 | 40 | 0.73 |
| with assistive device | 40 | 50 | 60 | 0.73 |
Median (range of values: min-max); nt: not tested; na: not applicable.
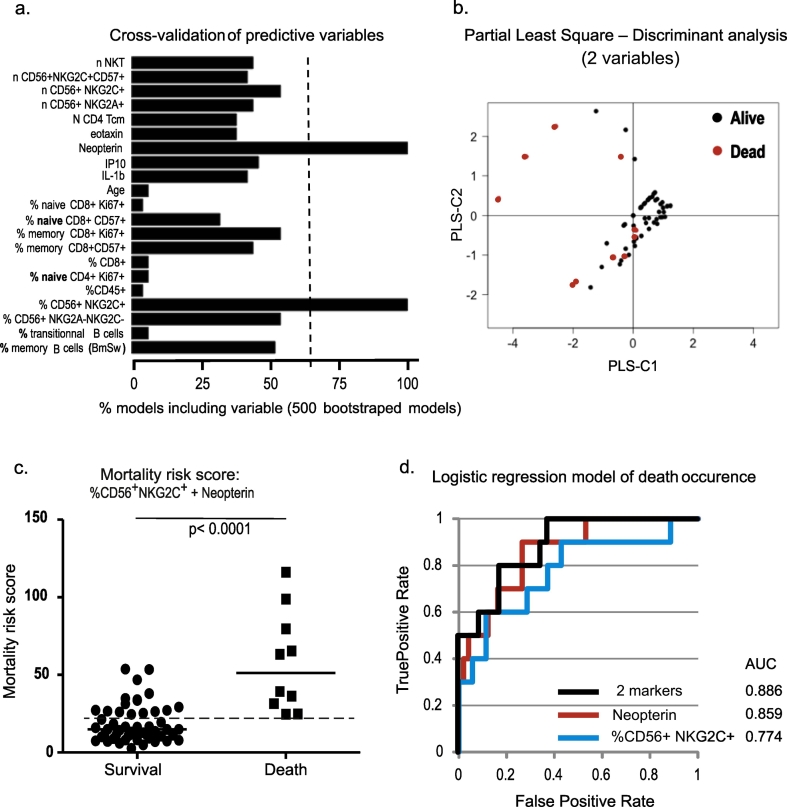
24 clinical parameters as well as 33 biological factors were measured and registered. We moreover assessed both cellular and plasmatic components of the immune system (n = 79). Our first aim was to identify biomarkers predictive of clinical outcome. We therefore analyzed our pre-surgery data exhaustively using a regularized logistic regression model with lasso penalty followed by 10-fold cross-validation of variables. Among the parameters collected at D0 for each individual, only 21 were found to distinguish between hip-fracture patients displaying good (survival; n = 50) versus bad (death; n = 10) clinical outcome (Fig. 2A). Only 2 of these 21 variables were outstanding (occurring in > 2/3 of the sparse models) and therefore kept for further analysis: 1) the percentage of CD16+ CD56dimNKG2C+ NK cells (defined according to the gating strategy depicted in Supplementary Fig. 1) and 2) plasmatic neopterin concentration. Using a Partial Least Square Discriminant analysis (PLS-DA) these two variables enable us to discriminate between the two subgroups (Fig. 2B). Indeed, the primary driver of the first PLS component (PLS-C1) explains 41.2% of the data variance discriminating survivors from non-survivors. Employing the loading scores obtained from the PLS-DA, each parameter contributes equally to PLS-C1 (Fig. 2B). Indeed, we can determine a survival score (PLS-C1) corresponding to the sum of the plasmatic neopterin concentration and the percentage of CD16+ CD56dimNKG2C+ NK cells. Contrarily, the second PLS component (PLS-C2) only explains 0.2% of the descriptive capacity and can therefore practically be neglected. Employing an appropriate cut-off value this survival score discriminates clearly between survival and death (Fig. 2C; p < 0.0001). We identified a cut-off value of 24.7 giving a test with 100% sensitivity and 70% specificity. Alternatively a cut-off value of 31.2 gave a test with 80% sensitivity and 84% specificity.
Fig. 2.
Predictive score of mortality post hip-fracture.
(a) Variables measurable prior to surgery capable of predicting death were identified using a lasso-regularized logistic regression model with 10-fold cross validation. To render the analysis more robust we repeated the model fitting (n = 500). Bar diagram depicts the frequency of models identifying a given variable. Only variables being identified in 2/3 of the models were selected for further analysis (dashed line).
(b) Scatter plot of a two components of a Partial Least Square Discriminant Analysis (PLS-DA) identifying the degree of variance of the predicted variable (death) explained by the two predictive variables identified by regularized logistic regression (neopterin concentration in nmol/L and % CD16+ CD56dimNKG2C+ NK cells). The explained variance is 41.2% and 0.2% for component 1 and 2 (PLS-C1 & PLS-C2), respectively. Symbols discriminate between alive (white symbols) and dead (black symbols) hip-fracture patients one-year post-surgery.
(c) Scatter plot depicting a mortality score mathematically derived from the PLS component 1 stratified according to survival status. The mortality score is calculated as the sum of neopterin concentration (nmol/L) and the % CD16+ CD56dimNKG2C+ NK cells. A proposed cut-off value (24.7) is indicated with a dotted line; this gives a test with 100% sensitivity and 70% specificity. Statistical significance is calculated with a non-parametric Mann-Whitney test.
(d) Receiver operating characteristic (ROC) curves of logistic regression models of death occurrence predicted by the two predictive variables in combination or individually (blue line for % CD16+ CD56dimNKG2C+ NK cells at D0; redline for plasmatic concentration of neopterin (nmol/L) at D0 and black line for the 2 variables in combination). Area under curve (AUC) for each curve is indicated.
Logistic regression models of death occurrence with each variable individually show that mortality is best predicted by neopterin plasma levels (Fig. 2D; AUCneopterin = 0.859 vs AUC%CD16+CD56dimNKG2C+NK cells = 0.774). Moreover, when comparing the ROC curves in Fig. 2D, the combination of the 2 variables does not show a clear improvement of the prediction model (AUCcombined = 0.886) compared to neopterin alone. For these reasons, we choose to focus on the level of neopterin reached by the patients at their arrival to hospital.
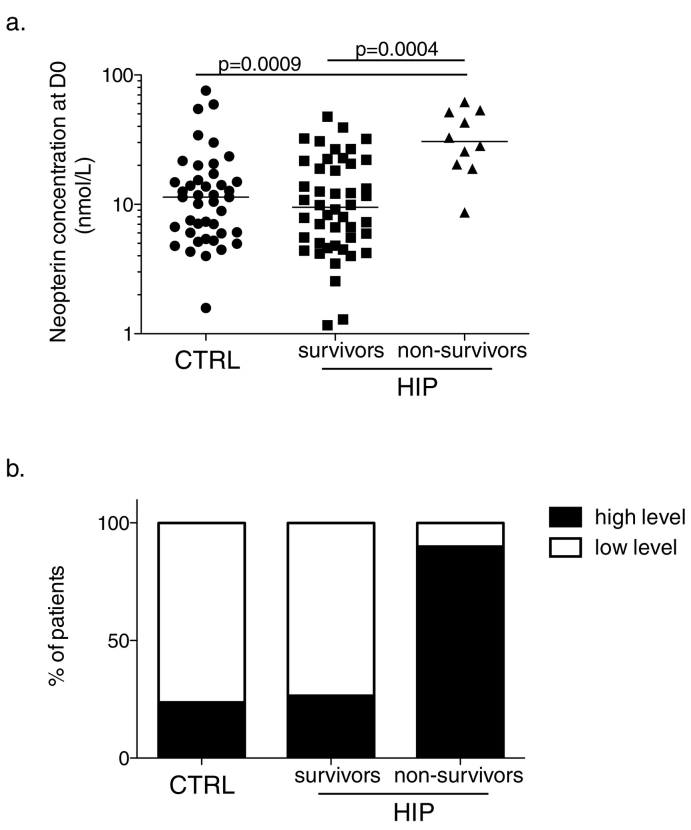
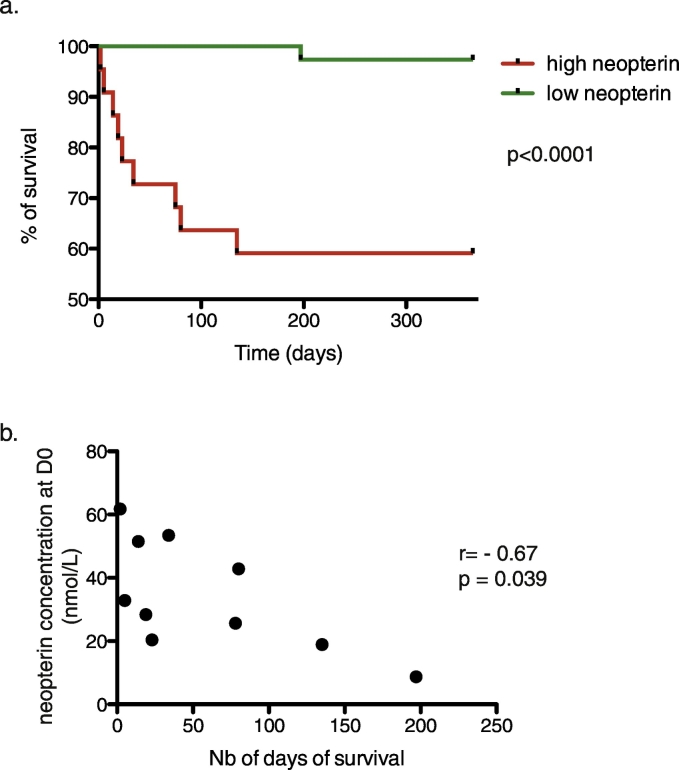
As shown in Fig. 3A, the concentration of neopterin at day 0 was similar between the control group of the healthy elderly and the hip-fracture patients, who survive post-fracture. However, statistical differences were observed when comparing hip-fracture patients who died within the first year post-fracture with either control individuals (p = 0.0009) or survivors (p = 0.0004). A cut-off value was set at 18.7 nmol/L, which corresponds to the upper 95% confidence interval of the mean of the neopterin concentration obtained from the cohort of control elderly included in our study (n = 42)(Fig. 3B). According to the level of neopterin measured in each patient at their arrival to hospital (high level > 18.7 nmol/L vs low level < 18.7 nmol/L), the probability of survival (within the year of follow-up) was statistically significant (Fig. 4A; p < 0.0001). The test has 90% sensitivity and 73.5% specificity.
Fig. 3.
Neopterin levels in elderly.
(a) The concentration of neopterin at arrival to hospital (in nmol/L) was measured for the control group of healthy elderly > 75 years old (black circles, n = 42) and was analyzed for the hip-fracture patients according to 2 clinical outcomes at one-year post fracture: survivors (black squares, n = 50) or non-survivors (black triangles, n = 10). p values are calculate with a non-parametric Mann-Whitney test.
(b) Distribution of the number of patients within each clinical subgroup (control healthy elderly/survivors post hip-fracture/non-survivors post hip-fracture), according to the upper 95% confidence interval of the mean of the neopterin concentration obtained from the elderly control cohort included in our study (n = 42). This value of 18.7 nmol/L separates individuals in two groups according to their neopterin level; low (white bar) vs high (black bar).
Fig. 4.
One-year post fracture survival.
(a) Kaplan-Meier curves showing the percent of survival one-year post fracture according to the level of plasmatic neopterin measured at D0, which corresponds to the time of arrival to the hospital. Statistical differences are assessed with Mantel-Cox test.
(b) Correlation between the level of neopterin measured at D0 (nmol/L) and the time of survival (days). Correlation statistics was assessed with a Spearman test. The coefficient of correlation is r = − 0.67; p = 0.039.
The specificity was particularly affected by 13 HFPs, exhibiting high neopterin levels (> 18.7 nmol/L) despite surviving. A penalized logistic regression analysis based on patients exhibiting high neopterin levels (13 survivors and 9 non-survivors) identified gender, IL-6 serum level and frequency of CD16+ CD56dimNKG2C+ NK cells as the parameters differentiating neopterin-high survivors from neopterin-high non-survivors (Supplementary Fig. 2A). Interestingly, the survivors display lower IL-6 levels than non-survivors (32.7 versus 10.9 pg/mL), suggesting that the survivors (dominantly women, 92% versus 50% in non-survivors) cope better with the acute stress and display a less inflammatory phenotype (Supplementary Fig. 2B).
Interestingly, from the non-survivors HFPs group, we could define a negative correlation between neopterin concentration at D0 and the number of days of survival after fracture (p = 0.039, r = − 0.67; Fig. 4B).
Of note, four clinical parameters were associated with neopterin: two positively-correlated (Dindo-clavien score and creatinine level) whereas two were negatively-correlated (albumin concentration and creatinine clearance (Cockroft)). However, statistical significances did not hold after corrections for multiple comparisons.
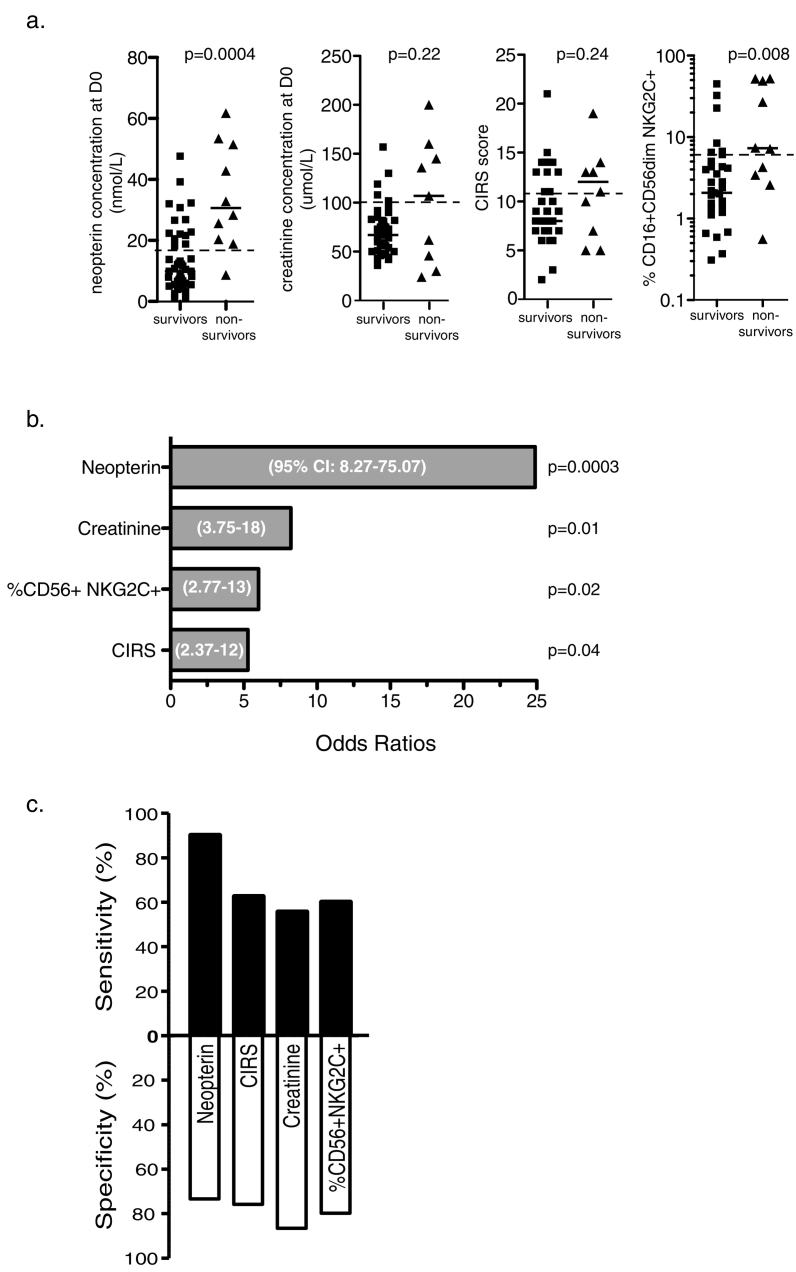
Other clinical and biological measurements, such as age, comorbidity (CIRS), low albumine (< 35 g/L), high creatinine (> 100umol/L), elevated procalcitonin (> 1μg/L) and IL-6 have been reported to be associated with various adverse clinical outcomes in HFPs. Despite the fact that those factors were not significant in our logistic regression analysis, we decided to compare, in our cohort, their predictive capacity to our newly identified predictor of one-year mortality, i.e. neopterin (Fig. 5A). We found that only 4 parameters exhibit significant odds ratios, neopterin being the best of them (ORneopterin = 24.9; p = 0.0003; ORcreatinine = 8.2, p = 0.01; ORCIRS = 7.7, p = 0.04; ORCD16+CD56dimNKG2C+NKcells = 6 p = 0.02) (Fig. 5B). Importantly, neopterin levels were also largely superior with regards to sensitivity (90%), while specificity was similar for all 4 parameters (Fig. 5C).
Fig. 5.
Predictive value of clinical, biological and immunological biomarkers.
(a) Scatters plots showing the distribution of clinical and biological parameters at D0 obtained from survivors (n = 50) vs non survivors (n = 10) post hip-fracture. From left to right: level of plasmatic neopterin, concentration of creatinine, comorbidity score (CIRS) and the frequency of NK CD56+ NKG2C+ cells. Dotted line represents threshold values for each individual variable.
(b) Histograms depicting odds ratios calculated for each variable based on threshold values indicated in (A): level of plasmatic neopterin (cut-off > 18.7 nmol/L), concentration of creatinine (cut-off > 100 μmol/L), comorbidity score (CIRS, cut-off > 11) and the frequency of NK CD56+ NKG2C+ cells (cut-off > 6%). Respective p-values and OR 95% confidence intervals of the individual logistic regression models are indicated on the graph.
(c) Stacked bars representing the sensitivity (black) and specificity (white) calculated from the variables distribution based on the threshold values employed in (B).
Neopterin should likely be considered a surrogate marker of post-surgery mortality as no causative link can presently be made with bad clinical outcome. Also, neopterin is not correlated with clinical parameters known to be associated with bad prognostic, although some weak but non-significant associations could be observed with levels of albumine, creatinine and creatinine clearance (Cockroft) as well as the Dindo-Clavien score.
In conclusion, these data demonstrate that neopterin is a robust predictive marker of mortality in elderly adults suffering from an acute hip-fracture. The concentration measured in the patients at their arrival to hospital is the best predictive marker observed in our experiments and correlated negatively with the time of survival after hip-fracture surgery. Predictive capacity may be slightly improved by including another marker, the percentage of CD16+ CD56dimNKG2C+ NK cells. Noteworthy, adding a known clinical indicator of fitness (time to walk post-surgery) as a third variable further improves the model (combined AUC = 0.951), although the latter parameter can only be determined post surgery and thus have less of a predictive capacity.
4. Discussion
Hip-fracture is an age-related health problem with substantially reduced long-term survival and autonomy (Beaupre et al., 2005, Hagino et al., 2010). The high mortality, particularly during the first year post surgery, is probably due to the combination of trauma, major surgery, low physiological reserve and concomitant medical problems. An elevation of inflammatory markers has been reported in these patients (Neumaier et al., 2006, Suzuki et al., 2004), which is regulated by host immune features, which could likely impact clinical outcome.
Here, we show that neopterin may serve as a predictive biomarker of one-year mortality post HFS. Pre-operative level of neopterin correlated with the number of days of survival and enable discrimination between patients at high risk of early death (i.e, within the first year post HFS) and survivors. Despite the limited number of patients included in this study, neopterin was clearly an outstanding parameter distinguishing patients with different clinical outcomes. However, larger studies are required to confirm this result and to potentially pinpoint other critical factors as biomarkers of one-year mortality after hip fracture.
Since neopterin is induced through IFN-γ secretion, its concentration might be considered as an indicator of systemic immune activation, reflecting in particular the activity of both innate myeloid cells (monocytes and macrophages) and of lymphoid cells (T and NK cells). Moreover, neopterin generation has been shown to correlate with reactive oxygen species (ROS) production (Nathan, 1986), suggesting neopterin as an indicator for oxidative stress (Murr et al., 1999).
Neopterin is biologically and chemically stable in body fluids resulting in accurate estimation of disease and hence prognosis. Furthermore, neopterin's measurement is easily applicable to the standards of medical laboratories. Indeed, it is a fast procedure that does not require high amount of biological material, nor specific high cost equipment requiring specialized personnel. This warrants the accessibility of this biomarker assessment to a large amount of potential patients.
To reduce mortality, attention must focus on optimizing health status pre-operatively (Ikpeze et al., 2017, Tarazona-Santabalbina et al., 2016), preventing post-operative complications (Liu et al., 2014), and, when the complications develop (Carpintero et al., 2014), providing optimal specialist medical care (Boddaert et al., 2014b, Boddaert et al., 2014a). Today, clinical decision-making post-HFS is mainly based on medical parameters since no biomarkers can be used to evaluate the risk for re-hospitalization or to decide on adapted care for frail patients. The expectation is that adjusting the level of care to the resilience status of older patients facing HFS will accelerate functional recovery, reducing the time spent at hospitals and the frequency of re-hospitalization, therefore having socio-economic benefits for public health care systems. In this respect, neopterin is a pivotal prognostic biomarker, which identifies patients at risk of morbidity with a very high sensitivity (90%). Indeed, a high sensitivity, which corresponds to the ability to identify all patients at risk of a bad outcome, is particularly important in a setting where the consequence of bad outcome is death. It is presently unknown if neopterin and associated hyper-inflammation is a surrogate immune correlate or if it has causal impact on disease progression. No study has specifically examined high-risk patients, who may gain most from more specialized medical care such as anti-inflammatory therapy. Further studies should identify optimal management, such as intensive physiotherapy or interventional treatment, for these patients aiming at a better resilience.
Of note, we propose that HFS may represent a model of acute stress in elderly. Therefore, knowledge from this study may open new avenues in our perception of other acute conditions, which represent endemic problems in aged patients. Whether other acute trauma or infections will show similar immune pattern warrants further work.
Acknowledgments
Acknowledgments
We are very grateful to the patients and staff of the department of geriatrics of the Pitié-Sâlpétrière Hospital. We acknowledge Anders Boyd for constructive scientific discussions and advices on biostatistics.
Funding Sources
This work was supported by the Fondation pour la Recherche Médicale (FRM) (Project DEQ20120323690) and the Agence Nationale de la Recherche (Project ANR-14-CE14-0030-01).
Conflicts of Interest
H.L., C.B. and J.C.B. declare no financial or commercial conflict of interest. M.L., V.A., J.B. and D.S. are inventors of patent EP16306120.3. M.L. is proprietary owner of the Funky Cells ToolBox software.
Author Contributions
M.L. analyzed the data and wrote the paper; C.B. performed experiments; H.L. recruited patients and analyzed clinical data; J.C.B. recruited patients and analyzed clinical data; V.A. designed research; J.B. designed clinical study, recruited patients and analyzed clinical data; D.S. designed research, performed experiments, analyzed the data and wrote the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.11.003.
Appendix A. Supplementary Data
Supplementary figures
References
- Bayard C., Lepetitcorps H., Roux A., Larsen M., Fastenackels S., Salle V., Vieillard V., Marchant A., Stern M., Boddaert J., Bajolle F., Appay V., Sauce D. Coordinated expansion of both memory T cells and NK cells in response to CMV infection in humans. Eur. J. Immunol. 2016;46:1168–1179. doi: 10.1002/eji.201546179. [DOI] [PubMed] [Google Scholar]
- Beaupre L.A., Jones C.A., Saunders L.D., Johnston D.W., Buckingham J., Majumdar S.R. Best practices for elderly hip fracture patients. A systematic overview of the evidence. J. Gen. Intern. Med. 2005;20:1019–1025. doi: 10.1111/j.1525-1497.2005.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloosesky Y., Hendel D., Weiss A., Hershkovitz A., Grinblat J., Pirotsky A., Barak V. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:420–426. doi: 10.1093/gerona/62.4.420. [DOI] [PubMed] [Google Scholar]
- Boddaert J., Cohen-Bittan J., Khiami F., Le Manach Y., Raux M., Beinis J.Y., Verny M., Riou B. Postoperative admission to a dedicated geriatric unit decreases mortality in elderly patients with hip fracture. PLoS One. 2014;9:e83795. doi: 10.1371/journal.pone.0083795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert J., Raux M., Khiami F., Riou B. Perioperative management of elderly patients with hip fracture. Anesthesiology. 2014;121:1336–1341. doi: 10.1097/ALN.0000000000000478. [DOI] [PubMed] [Google Scholar]
- Bouchon J.P. 1 + 2 + 3 ou comment tenter d'être efficace en gériatrie? Rev. Prat. 1984;34:888–892. [Google Scholar]
- Briza J., Kudrna K., Kvasnicka J., Busta O., Trca T. Acute phase reaction in severe injuries. Sb. Lek. 2002;103:193–202. [PubMed] [Google Scholar]
- Carpintero P., Caeiro J.R., Carpintero R., Morales A., Silva S., Mesa M. Complications of hip fractures: a review. World J. Orthop. 2014;5:402–411. doi: 10.5312/wjo.v5.i4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C.P., Lam Q.T., Ryan J.E., Sinnappu R.N., Lim W.K. Incidence of post-operative troponin I rises and 1-year mortality after emergency orthopaedic surgery in older patients. Age Ageing. 2009;38:168–174. doi: 10.1093/ageing/afn231. [DOI] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Fuchs D., Weiss G., Wachter H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int. Arch. Allergy Immunol. 1993;101:1–6. doi: 10.1159/000236491. [DOI] [PubMed] [Google Scholar]
- Gullberg B., Johnell O., Kanis J.A. World-wide projections for hip fracture. Osteoporos. Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- Hagino H., Sakamoto K., Harada A., Nakamura T., Mutoh Y., Mori S., Endo N., Nakano T., Itoi E., Kita K., Yamamoto N., Aoyagi K., Yamazaki K. Nationwide one-decade survey of hip fractures in Japan. J. Orthop. Sci. 2010;15:737–745. doi: 10.1007/s00776-010-1543-4. [DOI] [PubMed] [Google Scholar]
- Ikpeze T.C., Mohney S., Elfar J.C. Initial preoperative management of geriatric hip fractures. Geriatr. Orthop. Surg. Rehabil. 2017;8:64–66. doi: 10.1177/2151458516681145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings A.G., De Boer P. Should we operate on nonagenarians with hip fractures? Injury. 1999;30:169–172. doi: 10.1016/s0020-1383(98)00249-6. [DOI] [PubMed] [Google Scholar]
- Laulund A.S., Lauritzen J.B., Duus B.R., Mosfeldt M., Jorgensen H.L. Routine blood tests as predictors of mortality in hip fracture patients. Injury. 2012;43:1014–1020. doi: 10.1016/j.injury.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Le Cao K.A., Gonzalez I., Dejean S. integrOmics: an R package to unravel relationships between two omics datasets. Bioinformatics. 2009;25:2855–2856. doi: 10.1093/bioinformatics/btp515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn B.S., Linn M.W., Gurel L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Liu J., Ahn J., Elkassabany N.M. Optimizing perioperative care for patients with hip fracture. Anesthesiol. Clin. 2014;32:823–839. doi: 10.1016/j.anclin.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Murr C., Fuith L.C., Widner B., Wirleitner B., Baier-Bitterlich G., Fuchs D. Increased neopterin concentrations in patients with cancer: indicator of oxidative stress? Anticancer Res. 1999;19:1721–1728. [PubMed] [Google Scholar]
- Murr C., Widner B., Wirleitner B., Fuchs D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002;3:175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- Nathan C.F. Peroxide and pteridine: a hypothesis on the regulation of macrophage antimicrobial activity by interferon gamma. Interferon. 1986;7:125–143. [PubMed] [Google Scholar]
- Neumaier M., Metak G., Scherer M.A. C-reactive protein as a parameter of surgical trauma: CRP response after different types of surgery in 349 hip fractures. Acta Orthop. 2006;77:788–790. doi: 10.1080/17453670610013006. [DOI] [PubMed] [Google Scholar]
- Pimlott B.J., Jones C.A., Beaupre L.A., Johnston D.W., Majumdar S.R. Prognostic impact of pre-operative albumin on short-term mortality and complications in patients with hip fracture. Arch. Gerontol. Geriatr. 2011;53:90–94. doi: 10.1016/j.archger.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Ray N.F., Chan J.K., Thamer M., Melton L.J., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J. Bone Miner. Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.C., Muller M. pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinforma. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J.J., Wenn R.T., Sahota O., Moran C.G. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374. doi: 10.1136/bmj.38643.663843.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosencher N., Vielpeau C., Emmerich J., Fagnani F., Samama C.M. Venous thromboembolism and mortality after hip fracture surgery: the ESCORTE study. J. Thromb. Haemost. 2005;3:2006–2014. doi: 10.1111/j.1538-7836.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- Sedlar M., Kudrnova Z., Trca S., Mazoch J., Malikova I., Kvasnicka J., Krska Z., Zeman M., Linhart A. Inflammatory response in patients undergoing hip surgery due to osteoarthrosis or different types of hip fractures. Osteoarthr. Cartil. 2008;16:26–33. doi: 10.1016/j.joca.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Sedlar M., Kudrnova Z., Erhart D., Trca S., Kvasnicka J., Krska Z., Mazoch J., Malikova I., Zeman M., Linhart A. Older age and type of surgery predict the early inflammatory response to hip trauma mediated by interleukin-6 (IL-6) Arch. Gerontol. Geriatr. 2010;51:e1–6. doi: 10.1016/j.archger.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Seyedi H.R., Mahdian M., Khosravi G., Bidgoli M.S., Mousavi S.G., Razavizadeh M.R., Mahdian S., Mohammadzadeh M. Prediction of mortality in hip fracture patients: role of routine blood tests. Arch. Bone Joint Surg. 2015;3:51–55. [PMC free article] [PubMed] [Google Scholar]
- Sun T., Wang X., Liu Z., Chen X., Zhang J. Plasma concentrations of pro- and anti-inflammatory cytokines and outcome prediction in elderly hip fracture patients. Injury. 2011;42:707–713. doi: 10.1016/j.injury.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kawachi S., Sakai H., Nanke H., Morita S. Mini-incision total hip arthroplasty: a quantitative assessment of laboratory data and clinical outcomes. J. Orthop. Sci. 2004;9:571–575. doi: 10.1007/s00776-004-0830-3. [DOI] [PubMed] [Google Scholar]
- Tarazona-Santabalbina F.J., Belenguer-Varea A., Rovira E., Cuesta-Peredo D. Orthogeriatric care: improving patient outcomes. Clin. Interv. Aging. 2016;11:843–856. doi: 10.2147/CIA.S72436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet H., Chenevier-Gobeaux C., Villain C., Cohen-Bittan J., Ray P., Epelboin L., Verny M., Riou B., Khiami F., Boddaert J. Prognostic value of serum procalcitonin after orthopedic surgery in the elderly population. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:438–443. doi: 10.1093/gerona/glw097. [DOI] [PubMed] [Google Scholar]
- Zekry D., Loures Valle B.H., Graf C., Michel J.P., Gold G., Krause K.H., Herrmann F.R. Prospective comparison of 6 comorbidity indices as predictors of 1-year post-hospital discharge institutionalization, readmission, and mortality in elderly individuals. J. Am. Med. Dir. Assoc. 2012;13:272–278. doi: 10.1016/j.jamda.2010.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures