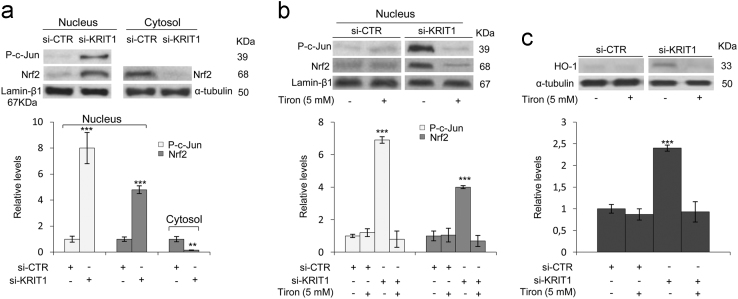
Fig. 4.
KRIT1 silencing in human brain microvascular endothelial cells causes a redox-dependent activation of the Nrf2 antioxidant response pathway. Human brain microvascular endothelial cells (hBMEC) grown under standard conditions were transfected with a KRIT1-targeting siRNA (si-KRIT1) or a scrambled control (si-CTR), and then either mock-treated or treated with the ROS scavenger Tiron (Tir, 5 mM for 24 h). Nuclear and cytoplasmic fractions (a,b) or total cell extracts (c) were then obtained and analyzed by Western blotting for Nrf2 (a,b) and HO-1 (c). Nuclear levels of p-c-Jun were used as a control of redox-dependent effect of KRIT1 loss-of-function [4]. Lamin-β1 and α-tubulin were used as internal loading controls for WB normalization of nuclear and total/cytoplasmic proteins, respectively. (a,b,c) The histograms below their respective Western blots represent the mean (± SD) of the densitometric quantification of three independent experiments. Notice that the upregulation of p-c-Jun nuclear levels induced by KRIT1 loss-of-function is paralleled by a marked nuclear accumulation of Nrf2 (a,b) and the upregulation of its downstream effector HO-1 (c), both of which are significantly reverted by cell treatment with the ROS scavenger Tiron (b,c).