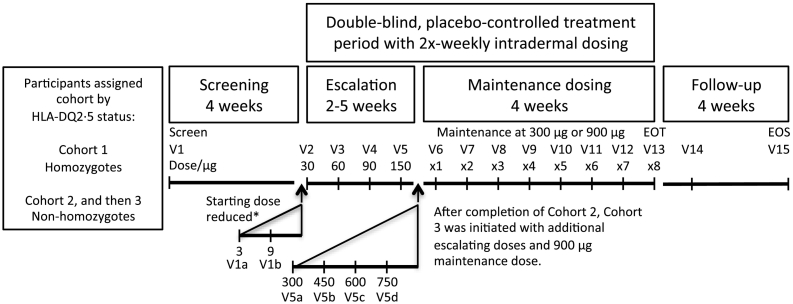
Fig. 1.
Study schematic. *Escalation was amended for all cohorts by including 3 μg and 9 μg doses when one participant in Cohort 1 withdrew with gastrointestinal adverse events graded moderate or severe after 30 μg and 60 μg doses. V14 was 1 week after V12. EOS, end of study; EOT, end of treatment; V, visit.