Abstract
Understanding the role of the environment in shaping the evolution of life histories remains a major challenge in ecology and evolution. We synthesize longevity patterns of marine sessile species and find strong positive relationships between depth and maximum lifespan across multiple sessile marine taxa, including corals, bivalves, sponges and macroalgae. Using long-term demographic data on marine sessile and terrestrial plant species, we show that extreme longevity leads to strongly dampened population dynamics. We also used detailed analyses of Mediterranean red coral, with a maximum lifespan of 532 years, to explore the life-history patterns of long-lived taxa and the vulnerability to external mortality sources that these characteristics can create. Depth-related environmental gradients—including light, food availability, temperature and disturbance intensity—drive highly predictable distributions of life histories that, in turn, have predictable ecological consequences for the dynamics of natural populations.
Keywords: comparative demography, Corallium rubrum, deep sea, integral projection models, life history, longevity
1. Introduction
From sequoias to small desert shrubs to naked mole rats, the study of species with unusually long lifespans has long fascinated evolutionary ecologists [1–4]. Understanding the conditions where extreme life histories dominate and the potential consequences of these singular strategies for the dynamics of natural populations is central to ecology and evolution, and may also have important implications for conservation biology [5]. However, to date ecologists have only found weak correlations between extrinsic factors and longevity, and we still have relatively few full demographic descriptions for extremely long-lived species.
In terrestrial landscapes, high longevities are common in relatively inhospitable environments characterized by low availability of resources, such as desert and alpine ecosystems [6–8]. However, some species of extreme longevity inhabit highly productive habitats (e.g. the coastal redwood), and climate or habitat factors generally provide only weak predictions of life-history traits [9–12]. The marine realm is perhaps a more promising setting to examine the external factors driving life-history variation than are terrestrial habitats, owing to the set of physical–chemical properties that lead to strong and predictable depth-related gradients at smaller spatial scales than are observed in other systems [13]. The main abiotic factors controlling bottom-up processes in marine communities (light, food availability, temperature and disturbance intensity) all generally decrease with depth (e.g. [14,15]); these factors are also often invoked as likely selective agents favouring short or long lifespans [16]. If environment plays a role in shaping the evolution of longevity, we, therefore, expect an increase of lifespan along depth gradients. Yet, quantitative analyses addressing this hypothesis are scarce.
In this study, we first explore longevity patterns of marine habitat-forming species, asking how predictable longevity is across depths and whether there are consistent consequences of individual longevity for population dynamics. We discuss these results in the light of similar analyses for terrestrial plant species. Next, we conduct a focused analysis of the iconic Mediterranean red coral, examining aspects of population biology of an extremely long-lived species that may be general for high-longevity species. Our findings represent an important step towards a better understanding on the role of environmental conditions as drivers of the evolution and maintenance of longevity and have implications for the conservation of dominant structural species found at deep habitats, where demographic data are scarce.
2. Material and methods
(a). Review of demographic studies
Life-history strategies are complex combinations of multiple traits that describe the timing and magnitude of the species' reproduction, growth and survival, which in turn generate life-history metrics such as longevity, generation time and net reproductive rate. For two reasons, we have focused here on longevity as a proxy for this broader diversity in life-history strategies. First, several comparative demography studies have shown that longevity is highly correlated to many other demographic traits; in particular, longevity is positively correlated to age at maturity and generation time and negatively correlated to reproductive output and growth [17,18]. Second, unlike many complicated life-history traits that require detailed demographic study to estimate, longevity can be estimated in quite simple ways and is widely reported, making it especially suited to broad-scale comparisons.
To construct our dataset, we searched the literature for studies reporting longevity estimates and demographic data by using several combinations of keywords. We restricted our analysis to marine sessile species and used the terms ‘marine sessile’, ‘coral’, ‘hexacoral’, ‘octocoral’, ‘macroalgae’, ‘seaweed’, ‘bivalve’ and ‘bryozoan’. These were combined with either ‘demographic model’ and ‘matrix model’ or ‘longevity’ and ‘lifespan’ in the Web of Knowledge. We found 144 suitable studies reporting data for 241 species (electronic supplementary material, table S9). We include studies of polycheates that form cemented tubes, and thus are effectively sessile, as well as studies of bivalves that are and are not strictly sessile.
Maximum longevity is estimated through different methodologies. Some studies directly report estimates of maximum observed lifespan using growth rings or geochemical analysis or both. Others report standard demographic data in the form of a matrix model or annual survival rates of adult individuals or colonies. The latter were used to derive potential maximum lifespan based on demographic simulations using the same methodology as for the red coral (see §2d(iii), Demographic analysis below). When demographic data were based on non-annual transitions, annual lifespan was calculated as Lann = L × 12/T, where Lann is the annual lifespan, L is the non-corrected lifespan value and T is the period described by each transition in months.
As a proxy for habitat, we used depth of occurrence, quantified by the maximum depth reported for the species. While mean or median depth would be more informative, accurate information on these central depths is not available for a wide range of species. Maximum depth is more commonly reported and likely to be correlated with a species' characteristic depth range. Maximum depths were obtained by searching in the literature including the keywords ‘depth’, ‘depth range’ and ‘maximum depth’ combined to the species scientific name (electronic supplementary material, table S9).
(b). Demographic variability using matrix population models
To quantify temporal variance in population growth, we used stochastic demographic models based on at least 5-year study periods (four annual transition matrices) for marine sessile species (n = 9) and terrestrial plants (n = 25) (electronic supplementary material, table S10). For each species, we simulated the fate of 1000 initial individuals for 1000 years, assuming equal probabilities for each reported annual transition, and computed changes in population size by dividing the total number of individuals at each time (t) by the previous number (t − 1). We then log-transformed these realized annual lambda values and computed their variance. Two long-term demographic studies on bivalve species reported only observed annual lambdas, thus we computed variance in population growth directly from these observed lambdas after a log-transformation.
(c). Statistical analysis
We used Pearson's and Spearman's rank correlations and linear models to assess the relationships between depth and longevity as well as longevity and population growth variability. We also explored multiple combinations of potential predictors and their interactions by fitting a set of multiple linear models and using Akaike information criteria corrected for small sample sizes to select the best models. First, a set of linear models was fitted to the whole longevity dataset to test for effects of depth while accounting for different methodological differences in longevity calculations and also to explore potential significant interactions between depth and taxonomic class. We also ran a separate set of models to test if other taxomonic levels (Phylum or Order) had more explanatory power or shifted the results. A second set of models was fitted using only the species for which estimates were based on demographic models. For these species, we explored which longevity definition (see §2d(iii), Demographic analysis below) had a better fit with maximum depth. Finally, to assess the effect of longevity on demographic variability, a third set of models was fitted to sessile species for which four or more annual transition models were available, setting variance in population growth as a response variable and maximum longevity, habitat and their interaction as potential predictors. In these and all other statistical tests, longevity was log-transformed for analysis to achieve more linear results.
(d). Study case: the Mediterranean red coral Corallium rubrum
(i). Natural history
Precious corals are long-lived species found in several seas across the world. The red coral Corallium rubrum is a long-lived octocoral of the Mediterranean Sea. It is a depth-generalist species commonly observed in the northwestern Mediterranean at shallower depths in overhangs and caves but its bathymetrical distribution can reach up to 1000 m deep [19]. Owing to the high value of its carbonate skeleton for the ornamental jewellery industry, C. rubrum has been intensively harvested for millennia. Exploitation—both legal and illegal—as well as ongoing climate change are current threats to this species [20–25].
(ii). Study area and demographic monitoring
A total of 1144 coral colonies from eight C. rubrum populations along the northwestern Mediterranean region were individually monitored using photographic techniques (n = 30 photoquadrats of 20 × 20 cm at each population) over periods ranging from 3 to 10 years between 2003 and 2011 (electronic supplementary material, table S1; see [26] for detailed information).
(iii). Demographic analysis
Based on the photographic time-series data, we could estimate size-dependent annual survival, fecundity and growth rates. We used a set of generalized mixed effects models (GLMMs) to describe annual survival and fecundity rates. Because the goal was to describe the general life history of the red coral, population and year were included in the models as random factors. Growth rates are extremely low for this species [27,28], thus a nonlinear growth/shrinkage model was fitted to data of initial and final colony sizes measured for 247 colonies in two populations over periods of 7–8 years. Owing to relatively rare ‘extreme shrinkage’ of some colonies, we divided the growth/shrinkage process into normal growth and extreme retrogression, each with their own mean change of size (see the electronic supplementary material). The growth/shrinkage rates model and the fixed effects from the survival and reproduction GLMMs, which included colony size as a fixed effect and population and year as random factors, were then used to construct a deterministic integral projection model (hereafter IPM). We analysed this model as a large matrix based on 80 colony size-classes (see [26]; electronic supplementary material for a full description on model construction).
Maximum potential lifespan was estimated by deterministic simulations using the demographic IPMs. Specifically, we simulated the fate of 100 individuals starting at the same stage and recording the number of years until 50% (mean lifespan) and 5% (maximum potential lifespan) of individuals were still alive. To explore the expected mean and maximum lifespan conditional on starting at different life-stages, we ran three set of simulations: starting with 100 recruits, starting with 100 of the smallest size-class adults (first reproductive individuals [29]), and starting with 100 largest size-class adults. In all results reported in the main text, we use adult maximum longevity. To explore how robust these longevity metrics are to changes in the dimension of the transition matrix derived from the IPMs, we computed all these metrics using matrices constructed with 10 to 200 size-classes.
To assess the elasticity of deterministic population growth to each vital rate, we performed a perturbation analysis of size-dependent survival, fecundity, and mean normal growth and mean extreme shrinkage, using an 80 size-class model. Each size-dependent vital rate was separately increased or decreased (by 1% and 5%). A mean demographic model was derived that included each perturbation and its deterministic population growth rate (dominant eigenvalue) was computed. Finally, the size-dependent elasticity of each vital rate to λ was calculated using:
where a is the vital rate, j the size-specific interval, λ the deterministic population growth and 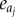 is the elasticity for vital rate aj. Since underlying vital rates were perturbed separately (e.g. mean growth, mean extreme shrinkage and survival), no compensation to control for shifting effects on other vital rates was required (see approach 4 in [30]).
is the elasticity for vital rate aj. Since underlying vital rates were perturbed separately (e.g. mean growth, mean extreme shrinkage and survival), no compensation to control for shifting effects on other vital rates was required (see approach 4 in [30]).
We calculated mean age-specific survival and fecundity rates by simulating a cohort of 1000 new recruits and computing at each time step the survival rate (Nt/Nt−1) and the fecundity (Rt/Nt−1), where N is the total adult abundance and R is the total number of recruits.
3. Results
(a). Depth, longevity and population stability
The comparison of maximum lifespan revealed a great diversity of life histories across marine sessile species (figure 1). Hydrozoans, bryozoans, cirripeds and polychaetes displayed the shortest lifespans, ranging from weeks to several decades. These generally short-lived taxa have received significantly less attention in the literature compared with other major taxa such as bivalves and corals. The other groups had a great range of lifespans, up to hundreds of years for some calcareous macroalgae and bivalves and to thousands of years for cnidarians and sponges. Most macroalgae species ranged from less than 1 year to several decades, but an outlier with exceptional longevity is the Antarctic calcareous algae Clathromorphum nereostratum. Marine bivalve's lifespans ranged from less than 1 year to more than 500 years for the Antarctic scallop Arctica islandica and the giant deep-sea oyster Neopycnodonte zibrowii, which is the longest-lived known non-clonal animal. Modular and structural taxa such octocorals, hexacorals and sponges were dominated by longer-lived species. Extreme cases were the deep-sea coral Gerardia sp., found in Hawaii, with an estimated age of 4700 years and the longest-lived known marine species, the sponge Monorhaphis chuni, observed at 1000 m depth with an estimated age of 11000 years [31,32].
Figure 1.
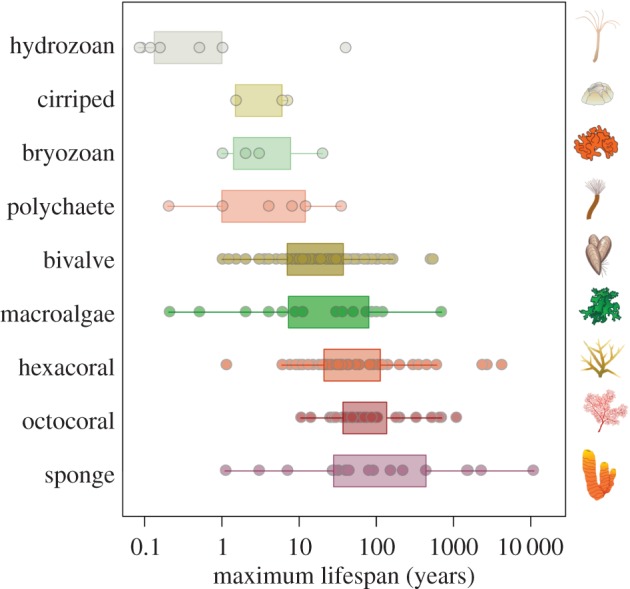
Longevity patterns across marine sessile species (n = 241). Data were obtained from the literature and correspond to the maximum potential lifespan reported for each species for the main marine sessile taxa. See the electronic supplementary material, table S9 for specific species data. (Online version in colour.) Images: Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/).
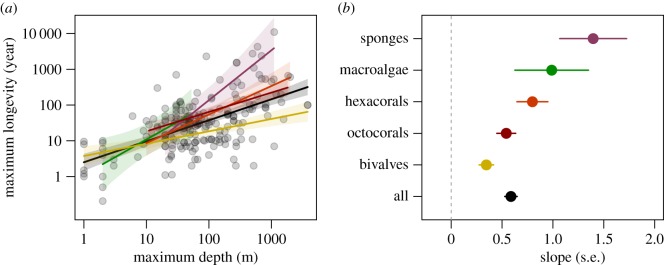
We correlated log(maximum lifespan) with log(maximum depth) and found strong positive relationships in the five main taxa for which enough data were available to perform meaningful analyses: octocorals (Spearman's ρ = 0.673; p < 0.001; n = 35), hexacorals (Spearman's ρ = 0.592; p < 0.001; n = 40), sponges (Spearman's ρ = 0.647; p < 0.001; n = 17), macroalgae (Spearman's ρ = 0.600; p = 0.023; n = 14) and bivalves (Spearman's ρ = 0.491; p < 0.001; n = 93) (figure 2; electronic supplementary material, table S2). The positive relationship between depth and lifespan was consistent across different measures of longevity, although adult maximum longevity showed the strongest relationships with depth (electronic supplementary material, table S3). This relationship is also consistent when accounting for taxon or matrix dimension; the best supported model includes an interaction between depth and taxon, with significantly larger slopes for sponges (p = 0.0131) and macroalgae (p = 0.0308) than for the other taxa (electronic supplementary material, table S4; overall model statistics: R2 = 0.511; F185 = 18.12; p < 0.001). Use of other taxonomic levels does not change this qualitative result, and model fit is best for Class (electronic supplementary material, table S5). We also ran quantile regressions between log(depth) and log(longevity) to see if depth is a more powerful regulator of extreme life-history values, but found no significant differences in the slope of the depth–longevity relationship for 10% up to 90% quantiles (electronic supplementary material, table S6).
Figure 2.
(a) Relationship between maximum depth of occurrence and maximum longevity across marine sessile species. Colours correspond to the labels in (b), and lines show the best fit linear model for each taxon. Black line shows the linear relationship for all taxa. Shaded areas represent standard errors. (b) Slope corresponds to the estimated slope of maximum depth on maximum longevity for each taxon, with error bars showing ±1 s.e. (Online version in colour.)
To assess population consequences of longevity, we regressed variance in realized log-lambda on longevity across marine sessile invertebrates and terrestrial plants and found a similar, strongly negative relationship for both. Short-lived species displayed higher variability in population growth over time than did longer-lived species (n = 34; Spearman's ρ = −0.73; p < 0.001). The pattern was consistent in terrestrial plants (n = 25, Spearman's ρ = −0.68; p = 0.0002) and marine sessile invertebrates (n = 9; Spearman's ρ = −0.67, p = 0.0214) (figure 3; electronic supplementary material, table S7).
Figure 3.
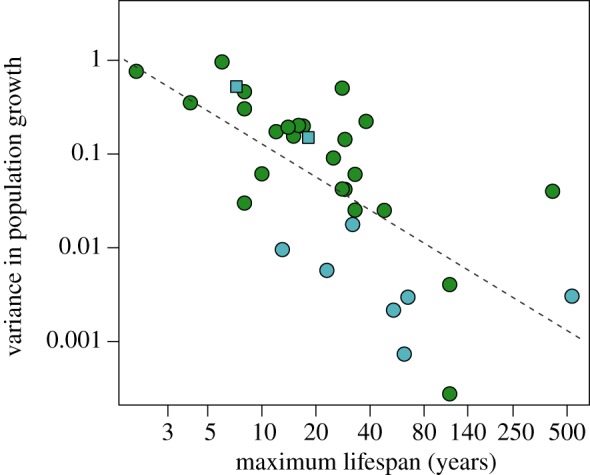
Relationship between temporal variability in population growth and maximum longevity in terrestrial plants (green, n = 25, Spearman's ρ =−0.68; p = 0.0002) and marine sessile (light blue, n = 9; Spearman's ρ =−0.67, p = 0.0214) species, and overall (n = 34; Spearman's ρ =−0.73; p < 0.0001). Squared light blue dots correspond to two bivalve species whose variance was computed directly from observed annual lambdas. (Online version in colour.)
(b). The extreme life-history of Corallium rubrum and its population dynamics
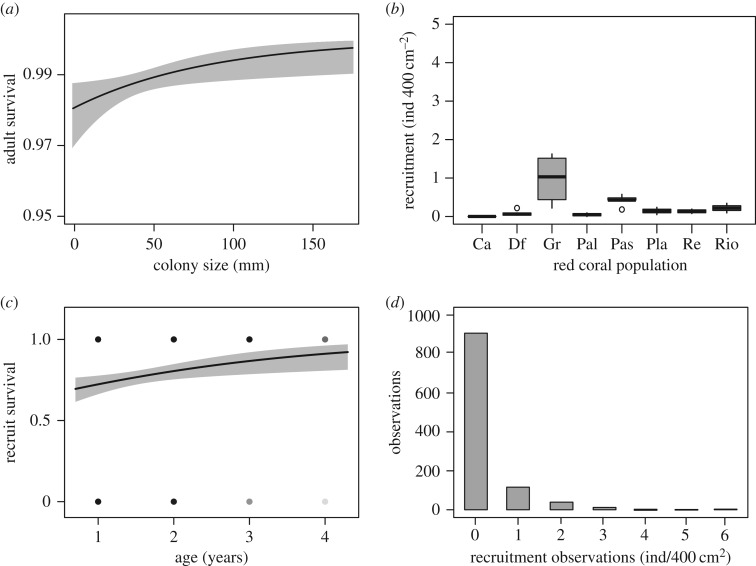
The red coral C. rubrum displayed slow dynamics over the eight populations studied along the northwestern Mediterranean (electronic supplementary material, figure S1), a pattern driven by extremely high adult survival, ranging between 98.05% and 99.76% from the smallest to the largest reproductive C. rubrum colonies (figure 4a). Monitoring of post-recruitment survival revealed that relative to later survival rates, annual survival is low during the first years after recruitment with a mean of 69.5% (figure 4c). However, by the age of four, young colonies already displayed a high survival probability of 92.2%. Overall, mortality rates decreased rapidly during the early stages of the red coral lifetime, following a type III survivorship curve, and then decreased steadily, more similar to type II survivorship (figure 5a). Recruitment was very limited; from 246 quadrats (about 30 per population) individually monitored for recruitment at the eight studied populations, 83.7% showed no annual recruitment. The remaining 16.3% of quadrats were divided into 10.7% with only a single recruit present, 5.1% with two to four recruits and only 0.5 with five or more recruits (figure 4b,d). Mean annual per capita recruitment, weighted by the relative abundance of different sized individuals, was 0.077. This is considerably lower than the average per capita recruitment for a range of marine sessile invertebrates for which there is standard demographic data available (n = 28, median = 0.547–0.727, mean = 5.14 ± 4.02 (s.d.); electronic supplementary material, table S8), although higher than the same estimate for six other species (electronic supplementary material, table S8).
Figure 4.
Long-term demographic traits of the red coral Corallium rubrum. (a) Adult survival probability depending on colony size. The line represents a logistic generalized linear mixed effects model including population and year as random effects and colony size as a fixed effect. (b) Mean recruitment rates in eight C. rubrum populations. (c) Age-dependent post-recruitment survival probability. (d) Frequency of annual recruitment observations in eight C. rubrum populations. In (a,c) grey areas show ±1 s.e.
Figure 5.
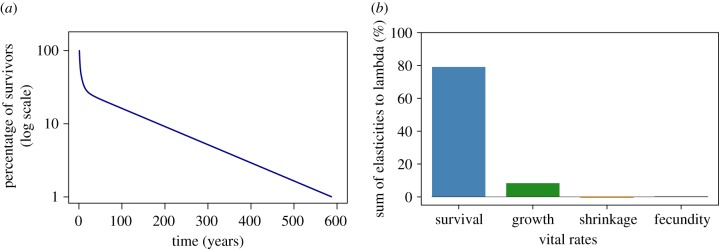
Demographic analyses for the red coral Corallium rubrum. (a) Survivorship curve based on a deterministic simulation of 100 individuals. (b) Patterns of elasticity to the asymptotic population growth rates of different vital rates, with each bar indicating elasticity summed across all size-classes. Other vital rates have even lower elasticities (electronic supplementary material, figure S4). (Online version in colour.)
Demographic simulations revealed high maximum potential future longevity for C. rubrum colonies, ranging from 501 to 532 years for small and large adult, respectively (figure 5a; electronic supplementary material, figure S2). The mean expected lifespan was also relatively high for adult red coral colonies and ranged from 98 years for small adult colonies to 129 years for the larger red coral colonies. Our simulations also revealed potential artefacts owing to matrix dimensionality. When using relatively small transition matrices (less than 60 size-classes) simulations underestimated values of maximum and mean longevity, the values stabilized around 60–80 size-classes and were consistent thereafter (electronic supplementary material, figure S2).
Perturbation analysis revealed a disproportionately large influence of survival rates on asymptotic population growth (figure 5b). Elasticity of lambda to fecundity, mean growth and mean extreme shrinkage, as well as all other vital rates, was markedly lower (electronic supplementary material, figure S4). Model fitting of age- or size-dependent fecundity and survival showed no evidence of senescence (electronic supplementary material, figure S3).
4. Discussion
Demography is a powerful tool to understand the evolution of life histories and has provided important insights into plant, mammal and bird's strategies [5,33]. However, a lack of long-term and broad spatial-scale data on the dominant species of marine ecosystems, particularly those living at deeper depths, hinders our understanding of the processes shaping the dynamics of these threatened ecosystems [34]. In this study, we analysed the long-term dynamics of the long-lived red coral C. rubrum and published data for a broad range of marine sessile invertebrates and macroalgae. The results revealed strong positive relationships between depth, longevity and demographic stability, showing strong habitat-determined predictability of key demographic processes in marine ecosystems.
Marine sessile species have a wide range of maximum lifespans across taxa and habitats, ranging from weeks in some intertidal species to hundreds of years for the red coral C. rubrum, and several thousands of years for some deep-sea corals and sponges (figure 1). Our most striking finding was that maximum lifespan was strongly and positively correlated with a species' maximum depth occurrence in hexacorals, octocorals, sponges, and although weaker, also in bivalves (figure 2). These results demonstrate a strong role of habitat features at shaping the distribution of longevity patterns.
In the shallowest waters, short-lived species dominate (figure 2), perhaps owing to high disturbance rates and competition in high-energy environments where fast-growing species have a distinct advantage. By contrast, species displaying diverse life-history strategies coexist at intermediate depths, including short-lived invertebrates that also occur intertidally and longer-lived massive corals, macroalgae and seagrasses [35,36]. The high energy availability owing to constant solar irradiation may allow the presence of a large spectrum of life-history strategies that have diversified to occupy multiple niches, following analogous processes than those shaping tropical rainforest plant communities [37]. Finally, in deeper waters, environmental constraints seem to favour the dominance of slower life histories and longer lifespans. Depth gradient determines changes in environmental factors such as light and food availability, temperature and disturbance intensities. In turn, these factors are likely to control the evolution of lifespans through several mechanisms. First, deeper habitats are generally less productive, limiting food supply for consumers and favouring slow growth and longer lifespans [38]. Empirical studies exploring physiological trade-offs have shown that the elevated metabolic costs of fast strategies can be detrimental in the absence of high availability of resources [39]. Also, as light is strongly depth-dependent, organisms dwelling at deeper habitats may experience a ‘competition release’, mainly by the absence of macroalgae and other fast-growing autotroph organisms that are constrained to the photic zone [40]. Similarly, deeper habitats are less diverse and thus organisms could face less predation pressure, pushing selective forces to favour somatic maintenance and, therefore, life extension [41]. Finally, the relative predictability of deep environments may enable species to survive with lower reproductive and mortality rates. Shallow habitats are more exposed to external sources of mortality owing to extreme physical disturbances (i.e. hurricanes and storms) and temperature fluctuations [13,42], making them less suitable for organisms whose long-term success relay on extremely high survival of adult stocks [43].
Previous anecdotal data on longevity in sessile and mobile marine organisms support our findings. For instance, the highest longevity reported to date corresponds to the sponge M. chuni, found at 1000 m depth with an estimated age of 11000 years [32]. A similar pattern has been also observed in marine fishes [44]. Indeed, a recent study based on radiocarbon dating on eye tissue revealed that the deep-resident Greenland shark Somniosus microcephalus, which can live up to 400 years, may be the longest-lived vertebrate on the Earth [45]. Overall, these results demonstrate a strong role of depth-related environmental gradients in shaping life-history strategies across marine taxa. The strength of this pattern is striking and contrasts with the weak environmental correlates of longevity or other life-history traits that seem typical in terrestrial systems [9–12]. Nonetheless, our findings open new questions regarding the existence of potentially analogous processes driving the evolution of life-history strategies along elevation and/or productivity gradients in terrestrial landscapes. Further analyses exploring the relationship between depth and different environmental parameters could provide a more mechanistic understanding of this general pattern of increasing species maximum longevity towards deeper marine waters.
At least three demographic mechanisms may contribute to the strong relationship observed between depth and longevity in marine sessile organisms. First, species may show relatively indiscriminate settlement. Under this scenario, the longevity pattern may arise because faster-growing, shorter-lived species outcompete slow species in productive, shallow sites, while at deeper sites only slow-growing species with high longevity may persist. Second, species may have evolved specific settlement cues so that larvae of long-lived species preferentially settle in either deep or shallow sites. Finally, to some extent these life-history patterns could result from demographic compensation, reflecting phenotypic plasticity in life histories (i.e. fecundity, growth or survival rates) as a function of local constraints on population growth rates [46]. In reality, all three mechanisms are likely to contribute to this matching of life history to environment.
(a). The extreme life-history features of the red coral Corallium rubrum
While our survey shows that mesophotic and deep-sea ecosystems are predictably inhabited by species of extreme longevity, we have few detailed studies of the population ecology of these taxa. We found that C. rubrum, a widely distributed Mediterranean red coral, showed a consistent pattern of slow population dynamics driven by extremely high survival, recruitment limitation and apparently negligible senescence (figure 5; electronic supplementary material, figure S3a). The species revealed a maximum potential longevity of up to 532 years, overlapping with lifespan ranges of other deep-sea organisms. Although previous research had shown that C. rubrum settlement can be highly heterogeneous [47,48], in this study, we observed a much more homogeneous pattern of low recruitment in the long term (figure 4b,d; electronic supplementary material, table S8). Recruitment limitation has been observed in other long-lived temperate invertebrates [49–51] and can seriously hinder the ability of populations to recover after intense perturbations [52].
For species with limited recruitment, adult survival becomes of paramount importance to ensure their persistence [43]. We found extremely high survival for C. rubrum (figures 4a and 5a), with estimated mortality even lower than found in previous experimental studies [27,50]. In agreement with previous observations in long-lived gorgonians [43], the perturbation analysis supported that survival is the vital rate demonstrating the highest effect on population growth in C. rubrum (figure 5b). The absence of detectable senescence is common in sessile marine species and terrestrial plants [2,53], and may be related to high investments in structural tissues, although some authors have suggested that modularity may also play an important role [53]. It should also be noted that our data, like that for most other studies of extremely long-lived species, lack the precision necessary to carefully test for senescence. Precious corals build an energetically demanding hard carbonate skeleton; while preventing a fast rate of colony growth, it may provide the structural basis that allows for an extremely high survival.
The shallow occurrences of the monitored populations of this long-lived species could seem to make it an outlier in the depth-longevity pattern. However, this coral is distributed from shallow to deep waters up to 1000 m depth [19] and its shallow occurrences are exclusively found in dim-light and dark habitats where it cannot be outcompeted by fast-growing organisms [40]. Given the overall pattern observed for marine species, the dynamics of red coral populations dwelling at deeper habitats are likely to be even slower and more dependent on adult survival. However, we must be cautious with these extrapolations and note that demographic data on deeper locations is not available owing to logistical constraints. Exceptional shallow presence has been also observed in other important cold-water corals, such as Lophelia pertusa, which can occur at 25 m in sediment laden fjords where light attenuation reduces the abundances of autotroph organisms, and thus competition; while at the other end of the scale this species may be found as deep as 3300 m [54].
(b). Population-level consequences of extreme life histories
As important as unravelling the drivers of extreme longevities are the implications of these strategies for dynamics of natural populations. We show that maximum longevity strongly predicts temporal stability in population growth across terrestrial and marine sessile species (figure 3). This finding agrees with previous studies suggesting that long-lived species can strongly buffer environmental stochasticity [55,56]. As observed for the red coral C. rubrum, this buffering capacity relies on high adult survival and low reproductive success. While conferring stability under natural conditions, this extreme life history will also hinder the ability of long-lived species to overcome increased mortality rates, but whether these species will be able to cope with rapidly changing perturbation regimes driven by the ongoing global change is still unclear. For instance, while the red coral has persisted after millennia of historical overharvesting owing to the combination of consistent survival of partially harvested colonies [25] and small size at maturity [29], new human-related stressors such as global warming have increased mortality rates and put some shallow populations at risk [20,21,57]. Worryingly, although declines may already be driving populations of some long-lived species towards collapse, in some cases, these trajectories may be too subtle to be noticed by ecologists and managers [58]. The consistent depth relationship for longevity also implies greater sensitivity of species and communities occurring at greater depths to human perturbations, amplifying recent calls to better monitor and protect these vulnerable ecosystems [34].
Supplementary Material
Acknowledgements
We thank K. Kaplan, M. Pages, A. Griffith and one anonymous reviewer for their valuable comments on early versions of this manuscript.
Data accessibility
All data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.p0b6b [59].
Authors' contributions
I.M.-S., C.L., D.F.D. and J.G. designed the study. C.L., J.B.L. and J.G. collected the demographic data in the field. I.M.-S. extracted data from the literature. I.M.-S. and D.F.D. performed analyses. I.M.-S. wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
This study was partially funded by the Spanish Ministry of Economy and Innovation Biorock project (CTM2009-08045), the Smart project (CGL2012-32194), the TOTAL Foundation Perfect project and the European Union's Horizon 2020 research and innovation programme under grant agreement no 689518 (MERCES). This output reflects only the authors' view and the European Union cannot be held responsible for any use that may be made of the information contained therein. I.M.-S. was supported by a FPI grant (BES-2013-066150), C.L. by a Ramon y Cajal (RyC-2011-08134) and J.B.L. by a postdoctoral grant (SFRH/BPD/74400/2010) from the Portuguese Foundation for Science and Technology. Support for D.F.D. came from National Science Foundation awards 1340024 and 1242355. I.M.-S., C.L., J.B.L. and J.G. are part of the Marine Conservation research group (2014 SGR 1297) from the Generalitat de Catalunya.
References
- 1.Buffenstein R. 2005. The naked mole-rat? A new long-living model for human aging research. J. Gerontol. A. Biol. Sci. Med. Sci. 60, 1369–1377. ( 10.1093/gerona/60.11.1369) [DOI] [PubMed] [Google Scholar]
- 2.Baudisch A, Salguero-Gómez R, Jones OR, Wrycza T, Mbeau-Ache C, Franco M, Colchero F, Hutchings M. 2013. The pace and shape of senescence in angiosperms. J. Ecol. 101, 596–606. ( 10.1111/1365-2745.12084) [DOI] [Google Scholar]
- 3.Jones OR, et al. 2014. Diversity of ageing across the tree of life. Nature 505, 169–173. ( 10.1038/nature12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colchero F, et al. 2016. The emergence of longevous populations. Proc. Natl Acad. Sci. USA 113, E7681–E7690. ( 10.1073/pnas.1612191113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith AB, Salguero-Gómez R, Merow C, McMahon S. 2016. Demography beyond the population. J. Ecol. 104, 271–280. ( 10.1111/1365-2745.12547) [DOI] [Google Scholar]
- 6.Vasek FC. 1980. Creosote bush: long-lived clones in the Mojave Desert. Am. J. Bot. 67, 246–255. ( 10.1002/j.1537-2197.1980.tb07648.x) [DOI] [Google Scholar]
- 7.Forbis TA, Doak DF. 2004. Seedling establishment and life history trade-offs in alpine plants. Am. J. Bot. 91, 1147–1153. ( 10.3732/ajb.91.7.1147) [DOI] [PubMed] [Google Scholar]
- 8.García MB, Pico FX, Ehrlén J. 2008. Life span correlates with population dynamics in perennial herbaceous plants. Am. J. Bot. 95, 258–262. ( 10.3732/ajb.95.2.258) [DOI] [PubMed] [Google Scholar]
- 9.Stearns SC. 1977. The evolution of life history traits: a critique of the theory and a review of the data. Annu. Rev. Ecol. Evol. Syst. 8, 145–171. ( 10.1146/annurev.es.08.110177.001045) [DOI] [Google Scholar]
- 10.McIntyre S, Lavorel S, Tremont RM. 1995. Plant life-history attributes: their relationship to disturbance response in herbaceous vegetation. J. Ecol. 83, 31–44. ( 10.2307/2261148) [DOI] [Google Scholar]
- 11.Morrison C, Hero JM. 2003. Geographic variation in life-history characteristics of amphibians: a review. J. Anim. Ecol. 72, 270–279. ( 10.1046/j.1365-2656.2003.00696.x) [DOI] [Google Scholar]
- 12.Moles AT, et al. 2007. Global patterns in seed size. Glob. Ecol. Biogeogr. 16, 109–116. ( 10.1111/j.1466-8238.2006.00259.x) [DOI] [Google Scholar]
- 13.Garrabou J, Ballesteros E, Zabala M. 2002. Structure and dynamics of north-western Mediterranean rocky benthic communities along a depth gradient. Coast. Shelf. Sci. 55, 493–508. ( 10.1006/ecss.2001.0920) [DOI] [Google Scholar]
- 14.Barnes RSK, Hughes RN.. 1999. An introduction to marine ecology. New York, NY: John Wiley and Sons. [Google Scholar]
- 15.Smith KF, Brown JH. 2002. Patterns of diversity, depth range and body size among pelagic fishes along a gradient of depth. Glob. Ecol. Biogeo. 11, 313–322. ( 10.1046/j.1466-822X.2002.00286.x) [DOI] [Google Scholar]
- 16.Munch SB, Salinas S. 2009. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc. Natl Acad. Sci. USA 106, 13 860–13 864. ( 10.1073/pnas.0900300106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard JM, et al. 2016. Life history axes of variation. In Encyclopedia of evolutionary biology (ed. Kliman RM.), pp. 312–323. Oxford, UK: Academic Press. [Google Scholar]
- 18.Salguero-Gómez R, et al. 2016. Fast–slow continuum and reproductive strategies structure plant life-history variation worldwide. Proc. Natl Acad. Sci. USA 113, 230–235. ( 10.1073/pnas.1506215112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knittweis L, et al. 2016. New depth record of the precious red coral Corallium rubrum for the Mediterranean. Rapp. Comm. Int. Mer Médit. 41, 467. [Google Scholar]
- 20.Garrabou J, Perez T, Sartoretto S, Harmelin JG. 2001. Mass mortality event in red coral Corallium rubrum populations in the Provence region (France, NW Mediterranean). Mar. Ecol. Prog. Ser. 217, 263–272. ( 10.3354/meps217263) [DOI] [Google Scholar]
- 21.Garrabou J, et al. 2009. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Change Biol. 15, 1090–1103. ( 10.1111/j.1365-2486.2008.01823.x) [DOI] [Google Scholar]
- 22.Garrabou J, et al. 2017. Re-shifting the ecological baseline for the overexploited Mediterranean red coral. Sci. Rep. 7, 42404 ( 10.1038/srep42404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linares C, Garrabou J, Hereu B, Diaz D, Marschal C, Sala E, Zabala M. 2012. Assessing the effectiveness of marine reserves on unsustainably harvested long-lived sessile invertebrates. Conserv. Biol. 26, 88–96. ( 10.1111/j.1523-1739.2011.01795.x) [DOI] [PubMed] [Google Scholar]
- 24.Bramanti L, et al. 2013. Detrimental effects of ocean acidification on the economically important Mediterranean red coral (Corallium rubrum). Glob. Change Biol. 19, 1897–1908. ( 10.1111/gcb.12171) [DOI] [PubMed] [Google Scholar]
- 25.Montero-Serra I, et al. 2015. Harvesting effects, recovery mechanisms, and management strategies for a long-lived and structural precious coral. PLoS ONE 10, e0117250 ( 10.1371/journal.pone.0117250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero-Serra I, et al. 2017. Accounting for life-history strategies and timescales in marine restoration. Conserv. Lett. 1–9. [Google Scholar]
- 27.Garrabou J, Harmelin JG. 2002. A 20-year study on life-history traits of a harvested long-lived temperate coral in the NW Mediterranean: insights into conservation and management needs. J. Anim. Ecol. 71, 966–978. ( 10.1046/j.1365-2656.2002.00661.x) [DOI] [Google Scholar]
- 28.Marschal C, Garrabou J, Harmelin JG, Pichon M. 2004. A new method for measuring growth and age in the precious red coral Corallium rubrum (L.). Coral Reefs 23, 423–432. ( 10.1007/s00338-004-0398-6) [DOI] [Google Scholar]
- 29.Torrents O, Garrabou J, Marschal C, Harmelin JG. 2005. Age and size at first reproduction in the commercially exploited red coral Corallium rubrum (L.) in the Marseilles area (France, NW Mediterranean). Biol. Conserv. 121, 391–397. ( 10.1016/j.biocon.2004.05.010) [DOI] [Google Scholar]
- 30.Griffith AB. 2017. Perturbation approaches for integral projection models. Oikos 126, 1675–1686. ( 10.1111/oik.04458) [DOI] [Google Scholar]
- 31.Roark EB, Guilderson TP, Dunbar RB, Fallon SJ, Mucciarone DA. 2009. Extreme longevity in proteinaceous deep-sea corals. Proc. Natl Acad. Sci. USA 106, 5204–5208. ( 10.1073/pnas.0810875106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jochum KP, Wang X, Vennemann TWSB, Müller WE. 2012. Siliceous deep-sea sponge Monorhaphis chuni: a potential paleoclimate archive in ancient animals. Chem. Geol. 300, 143–151. ( 10.1016/j.chemgeo.2012.01.009) [DOI] [Google Scholar]
- 33.McDonald JL, Franco M, Townley S, Ezard TH, Jelbert K, Hodgson DJ. 2017. Divergent demographic strategies of plants in variable environments. Nat. Ecol. Evol. 1, 0029 ( 10.1038/s41559-016-0029) [DOI] [PubMed] [Google Scholar]
- 34.Danovaro R, et al. 2017. An ecosystem-based deep-ocean strategy. Science 355, 452–454. ( 10.1126/science.aah7178) [DOI] [PubMed] [Google Scholar]
- 35.Arnaud-Haond S, Duarte CM, Diaz-Almela E, Marbà N, Sintes T, Serrão EA. 2012. Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS ONE 7, e30454 ( 10.1371/journal.pone.0030454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM. 2012. Evaluating life-history strategies of reef corals from species traits. Ecol. Lett. 15, 1378–1386. ( 10.1111/j.1461-0248.2012.01861.x) [DOI] [PubMed] [Google Scholar]
- 37.Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310. ( 10.1126/science.199.4335.1302) [DOI] [PubMed] [Google Scholar]
- 38.Larson DW. 2001. The paradox of great longevity in a short-lived tree species. Exp. Gerontol. 36, 651–673. ( 10.1016/S0531-5565(00)00233-3) [DOI] [PubMed] [Google Scholar]
- 39.Reznick DN, Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425. ( 10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 40.Zabala M, Ballesteros E. 1989. Surface-dependent strategies and energy flux in benthic marine communities or, why corals do not exist in the Mediterranean. Sci. Mar. 53, 3–17. [Google Scholar]
- 41.Healy K, Guillerme T, Finlay S, Kane A, Kelly SB. 2014. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B 281, 20140298 ( 10.1098/rspb.2014.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bridge TC, et al. 2014. Depth-dependent mortality of reef corals following a severe bleaching event: implications for thermal refuges and population recovery. F1000Res 2, 187 ( 10.12688/f1000research.2-187.v3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linares C, Doak DF, Coma R, Diaz D, Zabala M. 2007. Life history and viability of a long-lived marine invertebrate: the octocoral Paramuricea clavata. Ecology 88, 918–928. ( 10.1890/05-1931) [DOI] [PubMed] [Google Scholar]
- 44.Cailliet GM, Andrews AH, Burton EJ, Watters DL, Kline DE, Ferry-Graham LA. 2001. Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 36, 739–764. ( 10.1016/S0531-5565(00)00239-4) [DOI] [PubMed] [Google Scholar]
- 45.Nielsen J, et al. 2016. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 353, 702–704. ( 10.1126/science.aaf1703) [DOI] [PubMed] [Google Scholar]
- 46.Villellas J, Doak DF, García MB, Morris WF. 2015. Demographic compensation among populations: what is it, how does it arise and what are its implications? Ecol. Lett. 18, 1139–1152. ( 10.1111/ele.12505) [DOI] [PubMed] [Google Scholar]
- 47.Bramanti L, Magagnini G, De Maio L, Santangelo G. 2005. Recruitment, early survival and growth of the Mediterranean red coral Corallium rubrum (L 1758), a 4-year study. J. Exp. Mar. Bio. Ecol. 314, 69–78. ( 10.1016/j.jembe.2004.08.029) [DOI] [Google Scholar]
- 48.Santangelo G, Bramanti L, Rossi S, Tsounis G, Vielmini I, Lott C, Gili JM. 2012. Patterns of variation in recruitment and post-recruitment processes of the Mediterranean precious gorgonian coral Corallium rubrum. J. Exp. Mar. Bio. Ecol. 411, 7–13. ( 10.1016/j.jembe.2011.10.030) [DOI] [Google Scholar]
- 49.Grigg RW. 1988. Recruitment limitation of a deep benthic hard-bottom octocoral population in the Hawaiian Islands. Mar. Ecol. Prog. Ser. 45, 121–126. ( 10.3354/meps045121) [DOI] [Google Scholar]
- 50.Teixidó N, Garrabou J, Harmelin JG. 2011. Low dynamics, high longevity and persistence of sessile structural species dwelling on Mediterranean coralligenous outcrops. PLoS ONE 6, e23744 ( 10.1371/journal.pone.0023744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kersting DK, Teixidó N, Linares C. 2014. Recruitment and mortality of the temperate coral Cladocora caespitosa: implications for the recovery of endangered populations. Coral Reefs 33, 403–407. ( 10.1007/s00338-014-1144-3) [DOI] [Google Scholar]
- 52.Hughes TP, Tanner JE. 2000. Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81, 2250–2263. ( 10.1890/0012-9658(2000)081%5B2250:RFLHAL%5D2.0.CO;2) [DOI] [Google Scholar]
- 53.Tanner JE. 2001. The influence of clonality on demography: patterns in expected longevity and survivorship. Ecology 82, 1971–1981. ( 10.1890/0012-9658(2001)082%5B1971:TIOCOD%5D2.0.CO;2) [DOI] [Google Scholar]
- 54.Squires DF. 1959. Results of the puritan-American museum of natural history expedition to western Mexico. Bull. Am. Museum Nat. Hist. 118, 371–431. [Google Scholar]
- 55.Morris WF, et al. 2008. Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89, 19–25. ( 10.1890/07-0774.1) [DOI] [PubMed] [Google Scholar]
- 56.Garcia MB, Dahlgren JP, Ehrlén J. 2011. No evidence of senescence in a 300-year-old mountain herb. J. Ecol. 99, 1424–1430. ( 10.1111/j.1365-2745.2011.01871.x) [DOI] [Google Scholar]
- 57.Cerrano C, et al. 2000. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol. Lett. 3, 284–293. ( 10.1046/j.1461-0248.2000.00152.x) [DOI] [Google Scholar]
- 58.Hughes TP, Linares C, Dakos V, van de Leemput IA, van Nes EH. 2013. Living dangerously on borrowed time during slow, unrecognized regime shifts. Trends Ecol. Evol. 28, 149–155. ( 10.1016/j.tree.2012.08.022) [DOI] [PubMed] [Google Scholar]
- 59.Montero-Serra I, Linares C, Doak DF, Ledoux JB, Garrabou J. 2018. Data from: Strong linkages between depth, longevity and demographic stability across marine sessile species Dryad Digital Repository. ( 10.5061/dryad.p0b6b) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Montero-Serra I, Linares C, Doak DF, Ledoux JB, Garrabou J. 2018. Data from: Strong linkages between depth, longevity and demographic stability across marine sessile species Dryad Digital Repository. ( 10.5061/dryad.p0b6b) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.p0b6b [59].