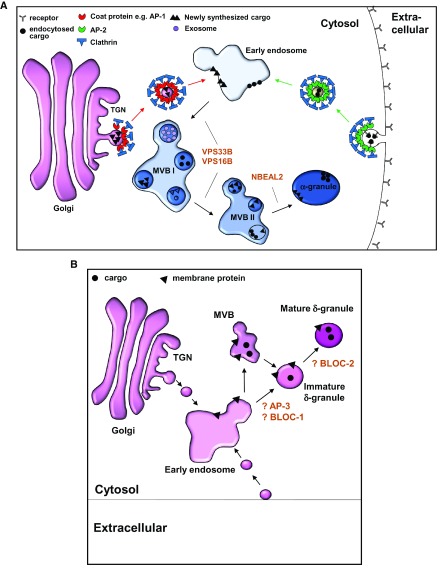
Figure 1. Working models of platelet α-granule and dense granule formation in megakaryocytes.
( A) α-Granules derive from two major pathways: synthetic and endocytic. The synthetic pathway originates at the trans-Golgi network (TGN). Soluble clathrin molecules recruited to the TGN self-assemble into a lattice structure and interact with coat proteins, presumed to be adaptor protein 1 (AP1), to form clathrin-coated pits. These pits invaginate to bud off early membrane-bound vesicles that are ultimately directed to early endosomes. Endocytic vesicles originate similarly at the plasma membrane employing adaptor protein 2 (AP2) and ultimately merge into early endosomes. α-Granules mature in multivesicular bodies (MVBs), a process that requires proteins VPS33B, VPS16B, and NBEAL2. ( B) Dense (δ) granules are lysosomal-related organelles, which are derived from the endosomal compartment. The current understanding of biogenesis of dense granule is highly speculative and was extrapolated from the biogenesis of melanosomes. Early endosomes provide input for developing dense granules, which may mature in MVBs. In melanosomes, BLOC1 is required for the exit of tubular structures carrying cargo from the endosomes, which are directed to the developing melanosomes by BLOC2. Alternatively, cargoes can be directed to developing dense granules by an AP3-dependent pathway, which may or may not require BLOC2. BLOC, biogenesis of lysosome-related organelles complex.