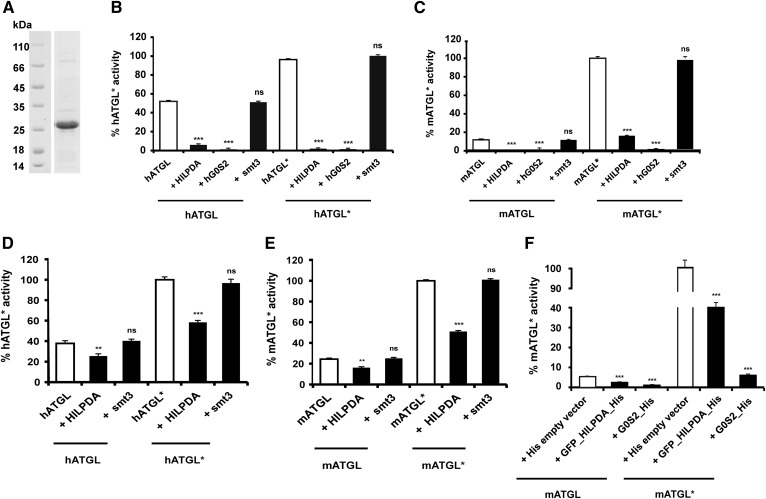
Fig. 2.
hHILPDA inhibits hATGL and mATGL under basal and CGI-58 stimulated conditions. A: Coommassie stained SDS-PAGE gel of purified smt3-HILPDA fusion protein (22 kDa). B–E: Activity assays were performed in the absence and presence (indicated by an asterisk, i.e., hATGL*) of 1 μg purified CGI-58. Twenty-five micrograms of ATGL lysates and 5 μg of purified HILPDA were used in all assays. Five micrograms of purified hG0S2 or 5 μg of purified smt3 were used wherever it is specified. Values corresponding to 100% ATGL activity upon activation with CGI-58 (i.e., hATGL*) are mentioned in parentheses below. TGH activity of bacterial cell lysates expressing human [343 nmol FA/h*mg protein (B)] or mouse [635 nmol FA/h*mg protein (C)] ATGL288 in the absence or presence of purified CGI-58, HILPDA, or smt3. TGH activity of HEK293 cell lysates expressing human [634 nmol FA/h*mg protein (D)] and mouse [638 nmol FA/h*mg protein (E)] ATGL in the absence and presence of purified CGI-58, HILPDA, or smt3. F: HEK293 cell lysates coexpressing mATGL and HILPDA were tested for TGH activity levels under basal and CGI-58-stimulated conditions. HEK293 cell lysates coexpressing mATGL (395 nmol FA/h*mg protein) and hG0S2 or empty vector served as controls. Statistical significance in comparison with ATGL* (white bar) was assigned according to the scheme: *P < 0.05, **P < 0.01, ***P < 0.001 representing three independent experiments. ns, not significant.