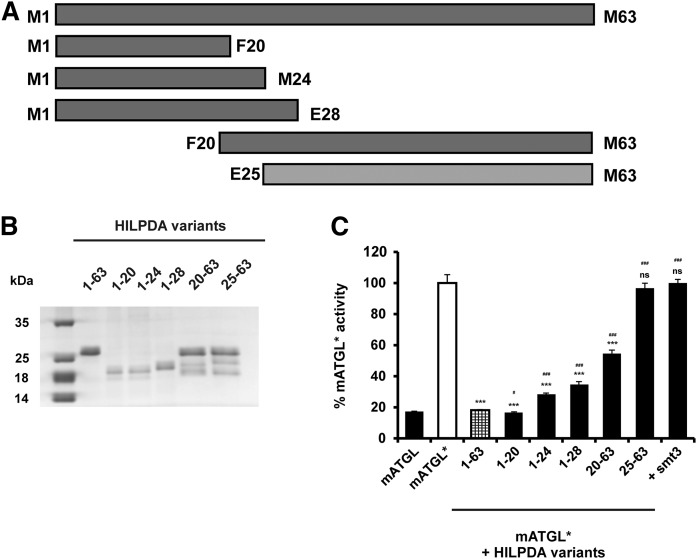
Fig. 3.
The N-terminal region of HILPDA is essential for ATGL inhibition. A: Graphical representation of full-length and C- and N-terminal truncated versions of HILPDA. Variants capable of ATGL inhibition are colored in dark gray. B: Coommassie stained SDS-PAGE gel of purified HILPDA variants expressed in E. coli. C: HILPDA variants were tested for their ability of ATGL inhibition. Activity assays were performed in the presence of CGI-58, as indicated by an asterisk (mATGL*). Five micrograms of purified HILPDA protein was mixed with 25 μg of mATGL288 lysate and 1 μg of purified mCGI-58. Purified smt3 was used as a negative control. One hundred percent ATGL activity corresponds to 635 nmol FA/h*mg protein. Statistical significance in comparison with ATGL* (white bar) was assigned according to the scheme: *P < 0.05, **P < 0.01, ***P < 0.001 and, in comparison with HILPDA_FL (checked bar), according to the following scheme #P < 0.05, ##P < 0.01, ###P < 0.001 representing three independent experiments. ns, not significant.