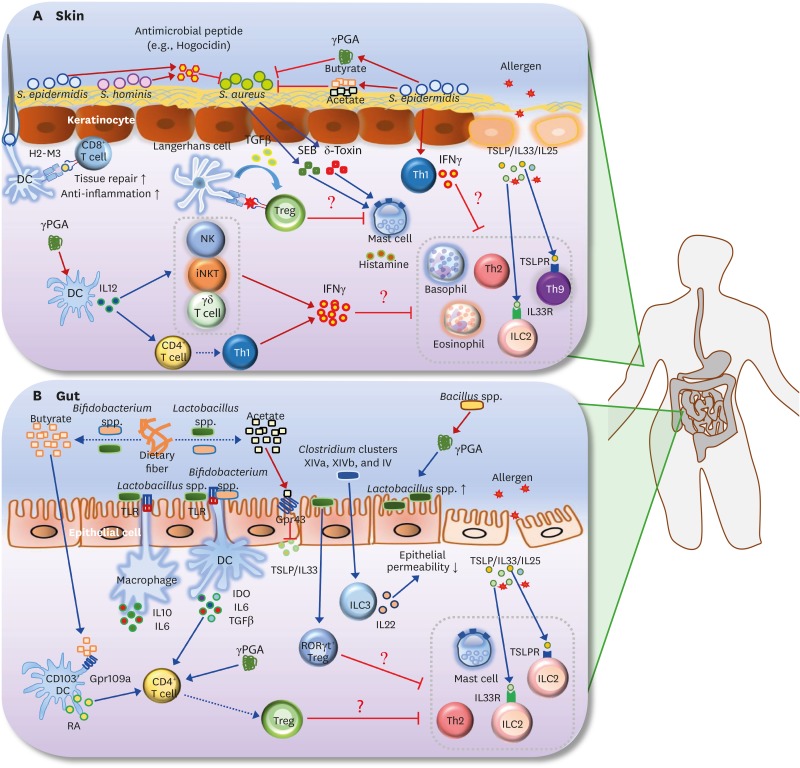
Figure 2.
Roles of the microbiota and its metabolites in AD and FA. (A) S. aureus is known to be the main pathogen that induces AD. SEB and δ-toxin, secreted by S. aureus, induce degranulation of mast cells in the skin. Survival of S. aureus in the skin is selectively inhibited by antimicrobial peptide (e.g., hogocidin) derived from commensal bacteria including S. epidermidis and S. hominis. S. epidermidis-derived γPGA suppresses survival of S. aureus. S. epidermidis also produces SCFAs (butyrate and acetate), which suppress the colonization of S. aureus in the skin. In addition, H2-M3-restricted commensal-specific CD8+ T cells, induced by S. epidermidis-stimulated DCs, contribute to both anti-inflammatory and tissue repair functions. The skin Treg population can be increased by the resting LCs via TGFβ; these Tregs may be responsible for the suppression of mast cell degranulation. Skin-resident commensal S. epidermidis increases IFNγ production by dermal T cells. In addition, γPGA from the skin commensal bacteria can activate DCs to induce differentiation of Th1 and activation of IFNγ-producing cells such as NK, iNKT, and γδ T cells. Consequently, IFNγ derived from S. epidermidis-activated Th1 cells, γPGA-induced Th1, and IFNγ-producing cells may lead to the suppression of skin allergic effector cells (e.g., ILC2, basophils, eosinophil, Th2 cells, and Th9 cells). (B) Butyrate and acetate are produced from dietary fiber by commensal bacteria including Lactobacillus spp. and Bifidobacterium spp. Acetate suppresses TSLP and IL33 via epithelial GPR43 signaling, and butyrate triggers CD103+ DCs to produce retinoic acid via GPR109a signaling. Lactobacillus spp. stimulates macrophages to produce IL10 and IL6 in a TLR2/TLR6-dependent manner. Moreover, commensal bacteria including Lactobacillus spp. and Bifidobacterium spp. induce differentiation of naive CD4+ T cells into Foxp3+ Tregs via IDO, IL10, and TGFβ. γPGA from gut commensal bacteria can directly induce the generation of adaptive Foxp3+ Tregs from naive CD4+ T cells. Gut extracellular γPGA, derived from Bacillus spp., induces compositional change of microbiota, such as increase of Lactobacillus spp. Non-toxin-producing Clostridium spp., including Clostridium clusters XIVa, XIVb, and IV decrease intestinal permeability via increased IL22 production by ILC3s. Lactobacillus spp. (e.g., Lactobacillus casei) promotes RORγt+ Treg differentiation in the gut. Thus, skin Tregs, induced by commensal microbiota and its metabolites (γPGA and SCFAs), can participate in the inhibition of gut allergic effector cells (e.g., ILC2, mast cells, and Th2 cells). Note that blue arrows indicate induction or stimulation, red arrows represent secretion, and red flat lines indicate inhibition. Moreover, dotted arrows indicate decomposition or differentiation.
SEB, S. aureus exotoxin B; TSLPR, TSLP receptor.