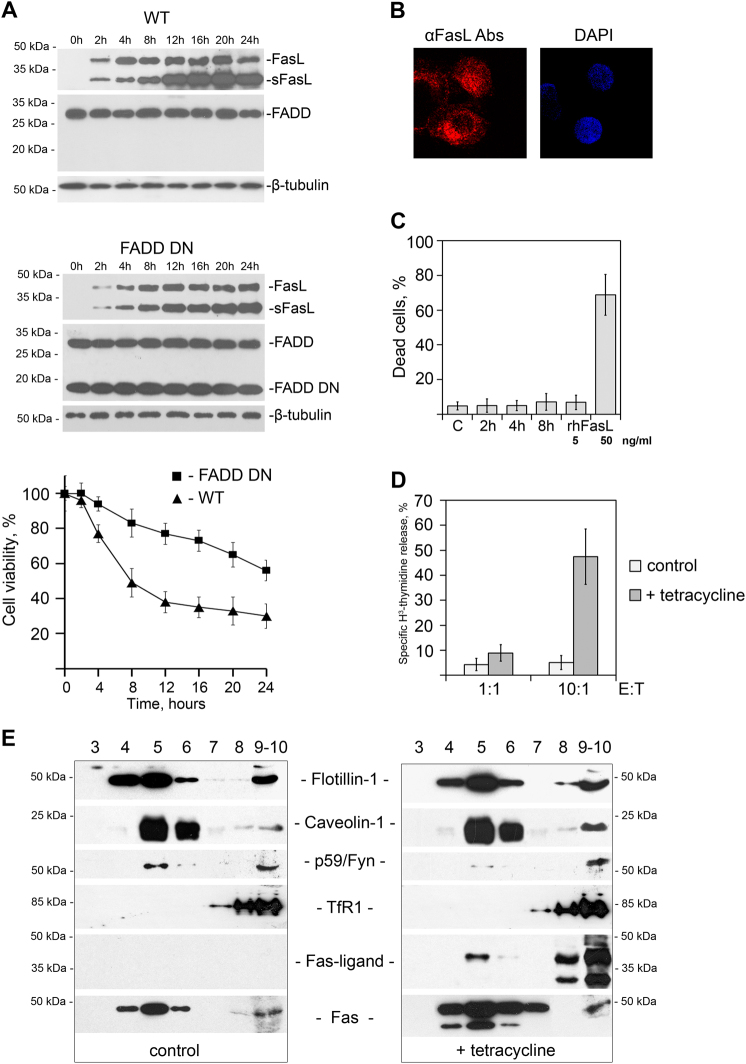
Fig. 1. Overexpression of FasL causes its translocation into rafts and induces cell death.
a Immunoblot analysis of FasL, FADD DN, and ß-tubulin expression in tetracycline-treated HeLa-pcDNA4/TO-FasL and HeLa-pcDNA4/TO-FasL–FADD DN cells in the specified time periods. FasL full-length Fas-ligand, sFasLshort form of FasL45, FADD Fas-associated death domain protein, FADD DN a dominant negative FADD mutant protein. β-tubulin is used as a loading control. Survival of HeLa cells overexpressing FasL alone (WT) (▲) and FasL together with the dominant negative FADD/MORT1 (FADD DN) (■), incubated with tetracycline for various time intervals (chart). Cell viability was determined by the neutral-red uptake method. Results are presented as means of at least three separate experiments, where error bars represent s.e.m. of biological triplicates. b HeLa-pcDNA4/TO-FasL cells were fixed and processed for immunolabeling for FasL (red) following by staining with the Alexa Fluor® 610-R-phycoerythrin goat anti-mouse Abs or DAPI (blue). c Survival of U937 cells incubated with HeLa-pcDNA4/TO-FasL condition medium collected 2, 4, and 8 h after tetracycline induction or 5 and 50 ng/ml of rhFasL (recombinant human FasL). Cell viability was determined via an MTT assay. d 3H-thymidine-labeled U937 cells were co-incubated with HeLa-pcDNA4/TO-FasL cells at various E:T ratios in the presence or absence of tetracycline. The percent of specific 3H-thymidine release from the target cells was determined after 12 h incubation. Results are presented as means of at least three separate experiments, where error bars represent SD of biological triplicates. e Lysates of non-induced (left panel) and tetracycline-induced for 4 h HeLa-pcDNA4/TO-FasL cells (right panel) were separated using ultracentrifugation in sucrose gradient. The distribution of raft-specific (Flotillin-1, Caveolin-1, p59Fyn) and non-specific (TfR1) markers as well as FasL and Fas was analyzed by immunoblotting