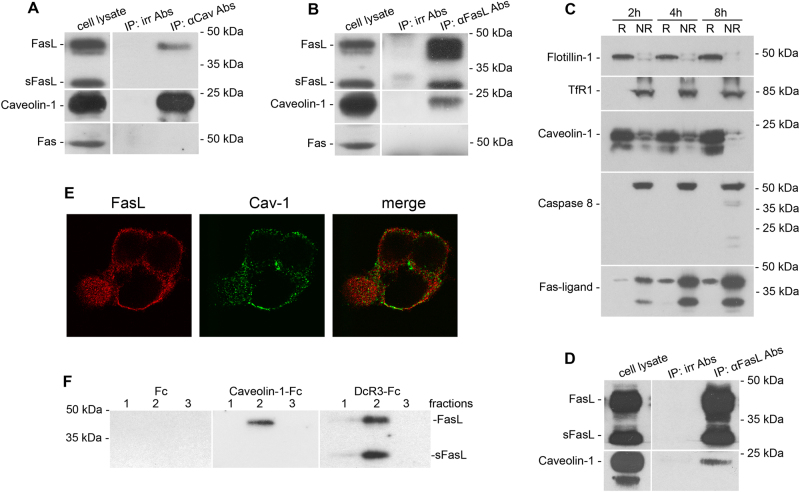
Fig. 2. FasL physically interacts with caveolin-1.
Lysates of HeLa-pcDNA4/TO-FasL cells induced by tetracycline for 4 h were immunoprecipitated with caveolin-1 Abs (a) or anti-Fas-ligand mAbs (b). Precipitates were analyzed by immunoblotting and stained with anti-Fas-ligand mAbs (a) or anti-caveolin-1 Abs (b), respectively. Both precipitates were analyzed by immunoblotting and stained with anti-Fas mAbs as well (lower panels of a and b). Irrelevant Abs were used as a negative control. c Immunoblot analysis of caveolin-1, caspase-8, and FasL expression in raft (R) and non-raft fractions (NR) of HeLa-pcDNA4/TO-FasL–FADD DN cells. Raft and non-raft fractions were prepared from lysates of tetracycline-induced for 2, 4, and 8 h HeLa-pcDNA4/TO-FasL–FADD DN cells. Flotillin-1 and TfR1 were used as raft-specific and non-specific markers, respectively. d Lysates of HeLa-pcDNA4/TO-FasL–FADD DN cells induced by tetracycline for 4 h were immunoprecipitated with anti-Fas-ligand mAbs followed by immunoblotting and staining with anti-caveolin-1 Abs. e Co-localization of FasL and caveolin-1. HeLa-pcDNA4/TO-FasL cells were fixed and processed for double immunolabeling for caveolin-1 (green) and FasL (red) followed by staining with the fluorescein goat anti-rabbit IgG and Alexa Fluor® 610-R-phycoerythrin goat anti-mouse Abs, respectively. f Interaction between recombinant caveolin-1 and FasL. Resins containing recombinant caveolin-1- Fc, DcR3-Fc (positive control), and Fc fragment of human Ig (negative control) were incubated with the lysate of tetracycline-induced HeLa-pcDNA4/TO FasL cells. Bound proteins were eluted and subjected to immunoblotting using anti-Fas-ligand mAbs