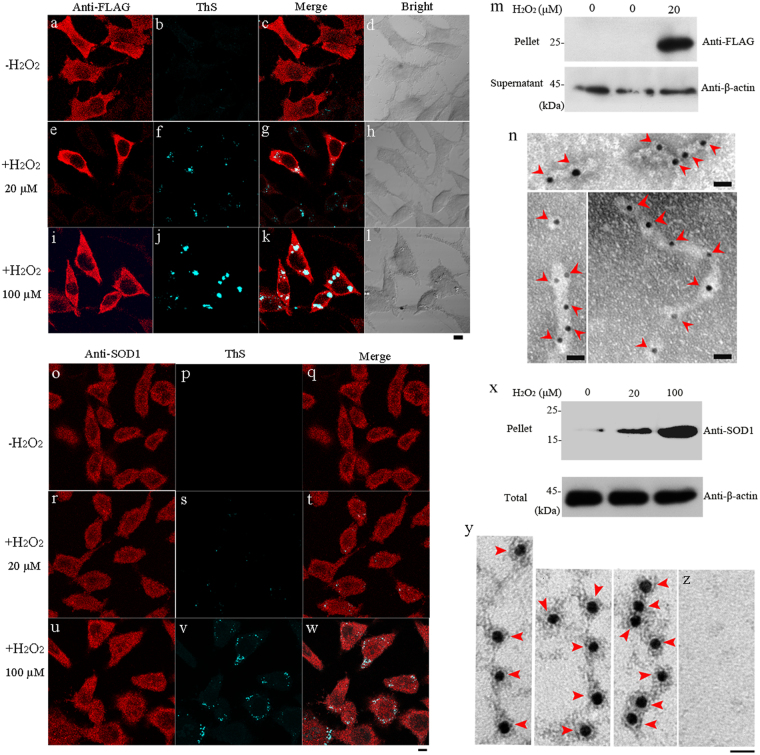
Fig. 2. Hydrogen peroxide at pathological concentrations triggers the fibrillization of wild-type human SOD1 in SH-SY5Y cells.
SOD1 stable cells treated with 20 (+) (e–h) or 100 μM (+) (i–l) H2O2, and SH-SY5Y cells treated with 20 (+) (r–t) or 100 μM (+) (u–w) H2O2, compared with SOD1 stable cells and SH-SY5Y cells treated without H2O2 (−) (a–c, o–q). SOD1 stable cells (a–l) and SH-SY5Y cells (o–w) were treated with 0–100 μM H2O2 at 37 °C for 4 and 6 days, respectively; fixed, ruptured and stained by thioflavin S (green), subsequently immunostained by mouse anti-FLAG antibody (a, e, i) or rabbit anti-SOD1 antibody (o, r, u) and homologous IgG conjugated with Alexa Fluo-546 (red), and observed with confocal microscopy. SOD1 stable cells (m) and SH-SY5Y cells (x) were treated at 37 °C with 0–20 μM H2O2 for 4 days and 0–100 μM H2O2 for 6 days, respectively, and then detected with western blotting. The Sarkosyl-insoluble pellets from SOD1 stable cells (m) and SH-SY5Y cells (x) were detected with mouse anti-FLAG antibody and rabbit anti-SOD1 antibody, respectively, and the supernatants from both cells were probed by mouse anti-β-actin antibody. H2O2 concentration from left to right (m) was zero (SH-SY5Y cells), zero (SOD1 stable cells), and 20 μM (SOD1 stable cells), respectively, and that from left to right (x) was zero (SH-SY5Y cells), 20 μM (SH-SY5Y cells), and 100 μM (SH-SY5Y cells), respectively. Untreated cells were the control (m, x, z). Aggregates extracted SOD1 stable cells (n) and SH-SY5Y cell line (y) incubated with 100 μM H2O2 were labeled by gold particles conjugated with anti-mouse/rabbit IgG. Red arrowheads were used to highlight amyloid fibrils. SOD1 fibrils were clearly observed by immunogold electron microscopy in both cases. Untreated SH-SY5Y cells were used as the control (z). The scale bars represent 10 μm (a–l),(o–w) and 20 nm (n, y, z), respectively