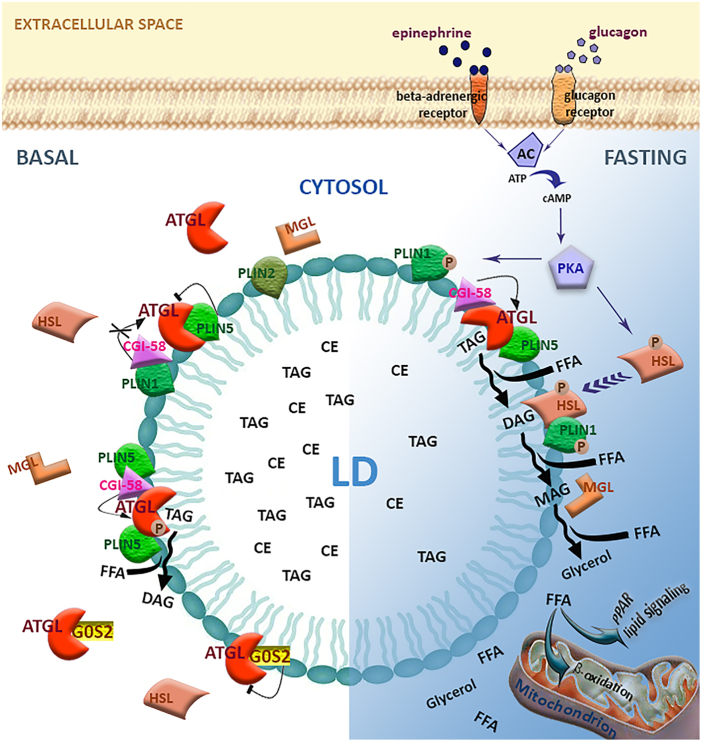
Fig. 1. Canonical lipolytic function of ATGL in adipocytes.
Schematic representation of lipid droplet (LD) components and the lipolytic machinery. LDs are organelles storing triacylglycerols (TAGs) and cholesteryl esters (CEs) and are composed of a phospholipid monolayer anchoring different proteins, including the perilipins (PLINs). In the unstimulated condition, ATGL is inhibited by the association with PLIN5 and G0S2. However, a basal TAG hydrolysis by ATGL could be achieved through AMPK-mediated phosphorylation and/or interaction with CGI-58, when the latter is not associated with PLIN1. In the fasted state, the hormones epinephrine or glucagon bind to their cognate receptors stimulating the adenylyl cyclase (AC) activity. Increased cAMP levels activate cAMP-dependent protein kinase (PKA), which phosphorylates ATGL, PLIN1 and hormone-sensitive lipase (HSL). Following PLIN1 phosphorylation, CGI-58 is released and thus it can further activate ATGL. PKA-mediated phosphorylation of HSL favours its association with LDs and its activity, leading to diacylglycerol (DAG) conversion into monoacylglycerol (MAG). The lipolytic cascade culminates with the MAG lipase (MAGL) activity, which induces the release of free fatty acid (FFA) and glycerol. FFAs are then delivered to mitochondria to produce energy through β-oxidation or used as signalling molecules for nuclear receptors, such as peroxisome proliferator-activated receptors (PPARs)