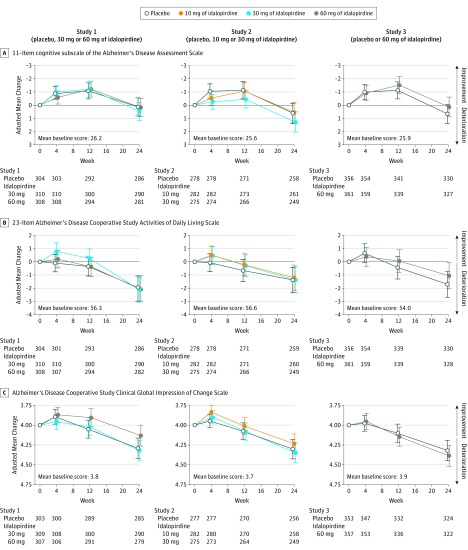
Figure 4. Primary and Key Secondary Efficacy Results in the 3 Studies.
Error bars indicate 95% CIs. The primary end point was change in cognition total score (range, 0 to 70; a lower score indicates less impairment) from baseline to 24 weeks as assessed by the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale. Key secondary end points were changes in activities of daily living (function) measured by the 23-item Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (total score range, 0-78; higher scores indicate less impairment) and overall clinical response (global outcome) measured by the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale from baseline to 24 weeks (rated from 1 [normal, not at all ill] to 7 [among the most extremely ill patients] at baseline; at subsequent visits, rated from 1 [very much improved] to 7 [very much worse]; 4 indicates no change). These end points were rated at baseline and at weeks 4, 12, and 24 (completion or withdrawal from the study). The targeted effect sizes for idalopirdine treatment was a difference of −2 points vs placebo for the cognitive subscale of the Alzheimer’s Disease Assessment Scale, a difference of 2 points vs placebo for the 23-item Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory, and a difference of −0.25 points vs placebo for the Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale.