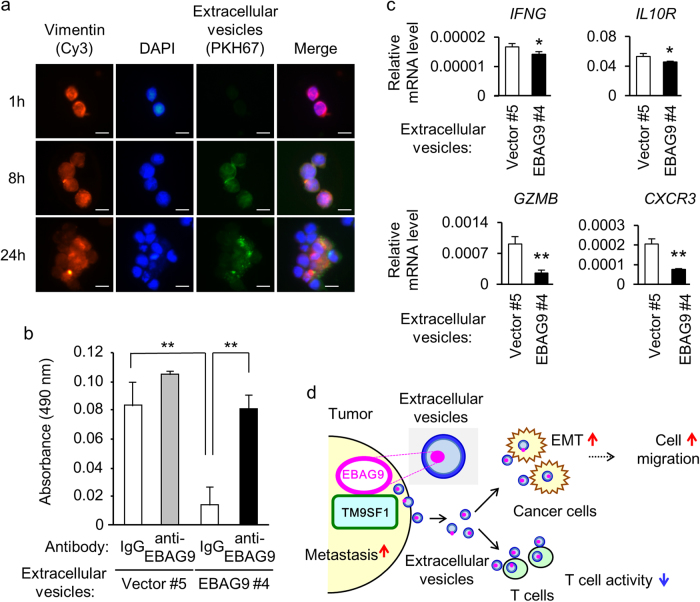
Fig. 6. EBAG9-containing extracellular vesicles (EVs) suppress T-cell cytotoxicity.
a Uptake of EVs into T cells. Fluorescent images of MOLT4 cells were taken at indicated times after co-culture with green fluorescent PKH67-labeled EVs prepared from LNCaP-EBAG9 #4 cells. Cells were co-stained with Cy3-labeled anti-vimentin antibody and 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 10 μm. b EBAG9 monoclonal antibody neutralizes EVs-mediated suppression of T cell cytotoxicity. EVs preincubated with anti-EBAG9 or IgG were washed with PBS and then co-cultured with MOLT4 cells for 2 days. Cytotoxicity was assayed using CytoTox 96 non-radioactive cytotoxicity assay in which lactate dehydrogenase (LDH) released into the media from damaged cells were quantified as absorbance at 490 nm. Data are shown as mean ± SD (n = 4). c EVs derived from LNCaP-EBAG9 cells downregulate immune-related genes in MOLT4 cells. qRT-PCR analyses for IFNG, IL10R, GZMB, and CXCR3 expression were performed using RNAs prepared from MOLT4 cells treated with EVs. Data are shown as mean ± SD (n = 3). *P < 0.05; **P < 0.01 (two-sided Student’s t-test). d A proposed model for the EBAG9-dependent regulation of tumor immunity and cancer cell migration in tumor microenvironment. EBAG9 interacts with TM9SF1 in tumor cells and regulates cancer cell migration and EMT. EBAG9-containing EVs also suppress cytotoxicity of T cells involved in microenvironment