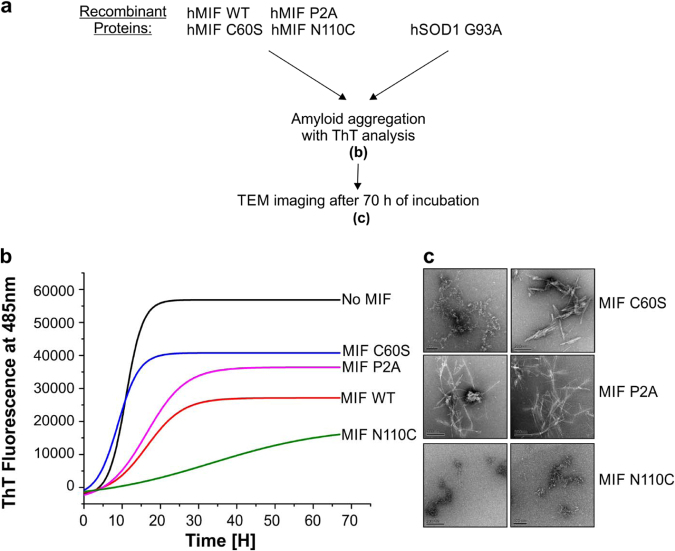
Fig. 5. The locked-trimeric mutant MIFN110C completely suppresses amyloid fibril formation of the mutant SOD1.
a Experimental protocol used for determining whether purified recombinant MIFWT or MIF mutants suppress the amyloid aggregation of misfolded SOD1, as detected by ThT and TEM . b ThT fluorescence was monitored during the incubation (37 °C with continuous shaking) of mutant SOD1G93A(50 µM), either without (black) or with recombinant MIFWT (red), MIFC60S (blue), MIFP2A(pink), or MIFN110C(green), all at a 10 µM concentration. Fluorescence was normalized to the maximal ThT fluorescence intensity that was elicited by SOD1G93A alone. Data indicate the average of 30–50 replicates performed from three independent experiments. Fluorescence was fitted to Boltzmann sigmoidal equation using OriginPro 8.5 software. c TEM images of a SOD1G93A (50 µM) solution after a 68-h incubation at 37 °C with continuous shaking. SOD1G93A was incubated in the presence of recombinant MIFC60S, MIFP2A, or MIFN110C at a molar ratio of 5:1.