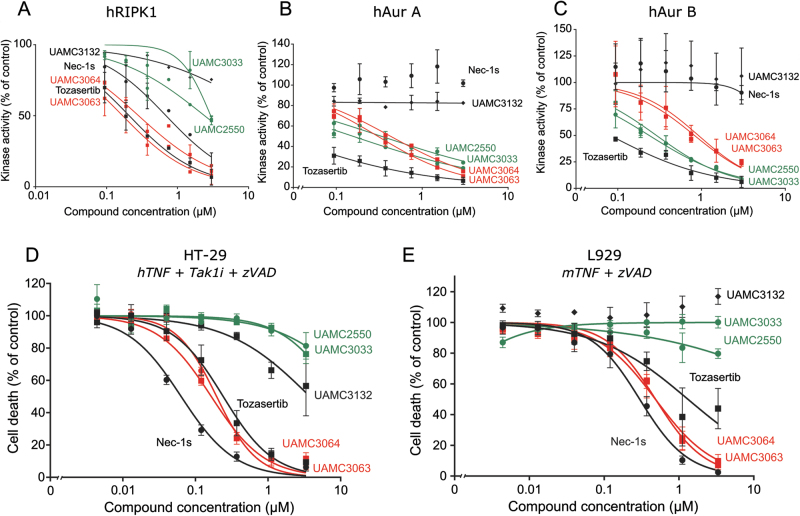
Fig. 3. Potency and specificity ranking of Tozasertib analogues is confirmed by in vitro kinase activity profiling and validated in a human and mouse cell line.
a–c An in vitro ADP-Glo kinase assay using recombinant human (h)RIPK1 (100 nM) (a), hAur A kinase (25 nM) (b) or hAur B kinase (25 nM) (c) was performed. Recombinant protein was incubated with the selected analogues, according to potency and specificity (selection indicated), at concentrations as indicated. Also, Nec1s and Tozasertib were included. Data represent mean value ±S.E.M. (n = 2). d Human HT-29 cells were pre-treated with Nec1s, Tozasertib and the Tozasertib analogues (concentration as indicated) for 1 h, followed by stimulation with hTNF (100 ng/mL) + Tak1i (1 µM) + zVAD.fmk (20 µM) for 17 h. Cells were stained with SytoxGreen (5 µM) and cell death was measured using the FluoStar fluorescence detection system. e Murine L929sAhFas cells were pre-treated with Nec1s, Tozasertib and the Tozasertib analogues (concentration as indicated) for 1 h, followed by stimulation with mTNF (20 ng/mL) for 3 h. Percentage cell death (% PI-positive nuclei) was determined as percent of control. d–e Data represent mean value ±S.E.M. (n = 3)