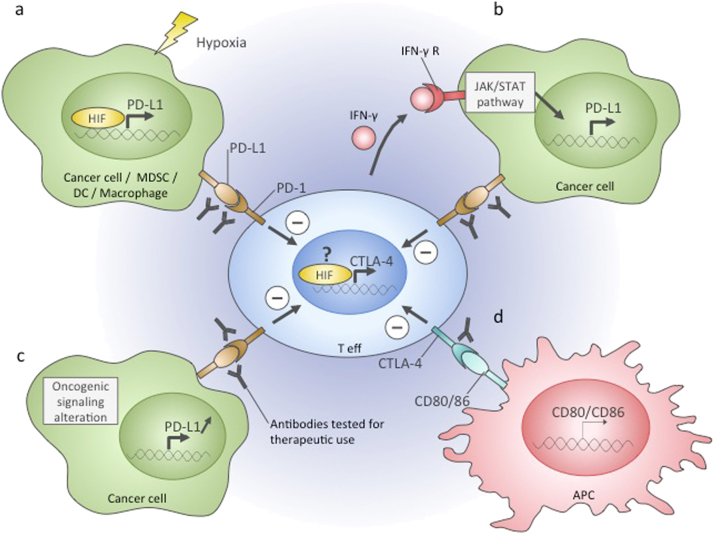
Fig. 3. Immune checkpoints in the tumour microenvironment.
. Effector T cells infiltrating the tumour can become repressed due to the activation of immune checkpoints. The targeting of PD-1 and CTLA-4 checkpoint pathways with specific antibodies is a promising therapeutic approach. In the tumour microenvironment, these pathways can be activated by the following mechanisms. a PD-1 receptor binding to its ligand PD-L1 leads to effector T-cell repression. PD-L1 can be expressed on the surface of cancer cells, MDSCs, DCs, and macrophages, and in these cells, it is directly transactivated by HIF in hypoxia. b Binding of interferon γ secreted by active effector T cells to its receptor on cancer cells results in activation of PD-L1 gene expression and subsequent T-cell repression. c PD-L1 gene expression can be constantly upregulated in cancer cells because of oncogenic mutations and signalling alteration, which leads to effector T cell repression upon interaction with this type of tumour cell. d Antigen-presenting cells express CD80 and CD86 ligands. Upon CD80/86 binding to CTLA-4 receptor on T cells, they become functionally repressed. Hypoxia was shown to induce CTLA-4 expression in T-cells, which potentially could contribute to their repression in the hypoxic tumour microenvironment. Teff effector T-cell, MDSC myeloid-derived suppressor cell, DC dendritic cell, IFNγ interferon gamma, IFN-γ R interferon gamma receptor, APC antigen-presenting cell