Highlights
-
•
Prognosis for patients with HCC and PVTT is historically poor.
-
•
Definitive RT may improve OS by prolonging the time to hepatic failure.
-
•
Our data suggest dose escalation may further improve OS.
-
•
Patients who received a BED >75 Gy had significantly better OS.
-
•
RT was well tolerated with no confirmed cases of radiation-induced liver disease.
Keywords: Hepatocellular carcinoma, Tumor thrombus, Prognostic factors, Dose escalation, Radiation therapy
Abstract
Background
The purpose of this study is to review the results of radiation therapy (RT) for hepatocellular carcinoma (HCC) with portal venous tumor thrombus (PVTT) in a Western patient population.
Methods
Thirty-four patients with HCC PVTT treated from 2007 to 2014 with RT were identified. Biologically effective dose (BED) was calculated for each patient, and greater than the median dose delivered (75 Gray (Gy)) was evaluated as a potential prognostic factor. Survival was compared and independent prognostic variables were evaluated by a Cox proportional hazards regression model.
Results
Twenty-six patients (76.5%) exhibited a radiographic response to RT, and 10 patients (29.4%) ultimately developed local failure. Local control, liver control, distant control and OS at one year were 57.1%, 36.4%, 55.2% and 57.4%, respectively. Patients who received a BED >75 Gy had a significantly better local control at 1 year (93.3% vs 45.6%; Log Rank p = 0.0184). Patients who received a BED >75 Gy also had significantly better median survival (24.7mo vs 6.1mo) and 1-year overall survival (76.5% vs 30.0%) when compared with BED ≤75 Gy (Log-Rank p = 0.002).
Conclusion
Our data suggest that RT should be considered for well-selected patients with HCC and PVTT for the purpose of improving local control and potentially prolonging the time to worsening venous obstruction and liver failure. When feasible, dose-escalation should be considered with a target BED of >75 Gy if normal organ dose constraints can be safely met.
Introduction
Patients with hepatocellular carcinoma (HCC) can develop portal vein tumor thrombi (PVTT) due to direct extension or by intravascular metastases. The incidence ranges from 12 to 44% at the time of diagnosis [1], [2]. Without treatment, patients with PVTT have a dismal prognosis with median survival rates of approximately three months [3], and fewer than one third of patients survive one year [4]. Currently, there is no consensus on how best to treat patients with HCC PVTT. United States [5] and European [6] guidelines recommend Sorafenib, while Asian consensus guidelines are more permissive of using locoregional treatments including surgical resection, radiation therapy (RT), transarterial chemoembolization (TACE), and radioembolization (RE) [7].
Initial concerns about radiation-induced liver disease (RILD) limited enthusiasm for RT in this patient population; however, as more data regarding dose-volume risk parameters become available [8], [9], [10], there has been increasing interest in the use of RT for patients with locally advanced or otherwise unresectable HCC, including those with PVTT [6], [7], [11]. Older studies suggested a potential, though modest, survival benefit with RT for these patients [12], [13], [14], [15], [16], [17]. More recently, advanced radiation techniques such as proton beam radiation (PBR) [18], [19], hypofractionated PBR [20] and stereotactic body radiotherapy (SBRT) [24], [25] have been utilized for normal tissue sparing, effective dose-escalation or both.
Most published studies have come from Asia, where there is a higher incidence of HCC [23]. However, the incidence of HCC is rising in Western countries given the increasing incidence of non-alcoholic steatohepatitis (NASH) [24]. To our knowledge, a dedicated investigation of definitive RT for HCC PVTT in a Western population has not been reported previously. Therefore, the purpose of this study was to evaluate the experience of a single institution in the use of radiation in the definitive treatment of HCC PVTT.
Methods
Patient selection
After institutional review board approval, we identified patients with pathologically or radiographically confirmed HCC with evidence of PVTT on ultrasonography or computed tomography (CT) treated with definitive EBRT at a single institution from 2007 to 2014. All patients were prescribed a Biologically Effective Dose (BED) of ≥45 Gy. The majority of patients received prior systemic therapy or liver-directed therapy with TACE or radiofrequency ablation; however, no patient received prior surgical intervention or prior radiation therapy. Concurrent sorafenib was sometimes given at the discretion of the treating medical oncologist with doses ranging from 200 mg daily to 400 mg twice daily.
Treatment
RT was delivered using either 3D conformal radiotherapy (3DCRT), intensity-modulated radiation therapy (IMRT), SBRT (≤5 fractions) or PBR based on physician preference. For patients receiving ≥50.4 Gy, daily image guidance included either daily CT-based alignment to soft tissue or kilovoltage xray-based alignment to liver fiducials in the inspiration breath-hold position [25], [26]. For all patients, the gross tumor volume (GTV) was delineated using all available imaging and included the PVTT plus the primary liver tumor and any radiographically involved lymph nodes if feasible to treat without unacceptable additional toxicity. A clinical target volume (CTV) was created to encompass potential microscopic disease by expanding the GTV by 0–10 mm. The planning target volume (PTV) was created by adding a 0–5 mm margin to the CTV. A central simultaneous integrated boost (SIB) dose of 60–100 Gy (2.4–5 Gy per fraction) was delivered to a volume created by contracting the GTV by 1 cm and subtracting a 5 mm planning risk volume (PRV) expansion around adjacent organs-at-risk (OARs) for select patients. The final dose and fractionation regimen was ultimately decided by the treating radiation oncologist. Our institutional practice from 2007–2010 was typically to use dose and fractionation regimens yielding a BED </=75 Gy (50.4 Gy in 1.8 Gy fractions, 45 Gy in 3 Gy fractions or 50 Gy in 5 Gy fractions, for example). After 2010, patients were sometimes offered dose and fractionation regimens yielding a BED >75 Gy (75 Gy in 3 Gy fractions or 67.5 Gy in 4.5 Gy fractions or 50 Gy in 12.5 Gy fractions, for example). In addition to temporal trends in our practice pattern, patients typically offered the most aggressive regimen achievable while meeting predetermined dose-volume constraints (Table 1). Acute toxicities were collected weekly and graded per the National Cancer institute Common Terminology for Adverse Events (NCI CTCAE) version 4.
Table 1.
Dose constraints for organs at risk utilized when treating hepatocellular carcinoma related portal vein tumor thrombi by daily radiation fraction size.
| Organ at risk | Dose constraint |
|---|---|
| 1.8–2.5 Gray fraction size | |
| Liver minus GTV | Mean <28 Gy (<24 Gy if Child-Pugh B) |
| Stomach/Duodenum/Small Bowel | Maximum <54 Gy |
| 3–4.5 Gray fraction size | |
| Liver minus GTV | Mean <24 Gy (<20 Gy if Child-Pugh B) 700 cc <24 Gy (<20 Gy if Child-Pugh B) |
| Stomach/Duodenum/Small Bowel | Maximum <45 Gy |
| ≥5 Gray fraction size* | |
| Liver minus GTV | Mean <16 Gy 700 cc <15 Gy |
| Stomach/Duodenum/Small Bowel | Maximum <28 Gy |
GTV = gross tumor volume, Gy = Gray.
Stereotactic radiation regimens in 3–5 total fractions.
Data collection
Pretreatment clinical features and details regarding prior systemic and local therapies were collected. Total radiation dose delivered was recorded both as nominal dose as well as BED, which was calculated using an α/β ratio of 10. All living patients were followed until August of 2016, and outcome measures including local, liver and distant control were collected as was vital status at last follow-up. Patients with metastatic disease at the time of radiotherapy were excluded from the distant recurrence analysis.
Statistical methods
Between-group comparisons were performed using the non-parametric Kruskal-Wallis test for continuous variables and the Pearson chi-square test for categorical variables. Survival times were calculated using the Kaplan-Meier methods from the point at which EBRT began. The log-rank test was used for statistical comparison of the survival curves for all potential variables. The Cox proportional hazards regression model was used by the forward stepwise method with all potential predictors with a p < 0.2 on univariate analysis were included in the multivariable model. Unadjusted P-values < 0.05 were considered to be significant. JMP® version 12 (SAS Institute Inc. Cary, NC) was used for all analyses.
Results
There were a total of 81 patients treated with RT for HCC between 2007 and 2014. Of these, 34 patients (42%) had PVTT confirmed on pre-radiation CT imaging. For these 34 patients included in this analysis, the median [range] follow-up was 12.8 [0.73–60.5] months. For patients alive at the time of the analysis, the median [range] follow-up was 18.7 [3.5–60.5] months. Patient characteristics are given in Table 2 and are separated by BED >vs ≤75 Gy (the median BED). Patients receiving a BED >75 Gy had a smaller gross tumor volume treated, received concurrent chemotherapy less often and received SBRT or PBR more often. Otherwise, baseline and treatment characteristics were similar.
Table 2.
Demographic, disease and treatment characteristics of patients treated with external beam radiation for hepatocellular carcinoma portal vein tumor thrombus.
| All patients (N = 34) | Patients treated with BED ≤75 Gy (N = 17) | Patients treated with BED >75 Gy (N = 17) | P-value | |
|---|---|---|---|---|
| Gender; N (column %) | .146 | |||
| Men | 29 (85%) | 16 (94%) | 13 (77%) | |
| Women | 5 (15%) | 1 (6%) | 4 (23%) | |
| Age at EBRT start in years; | .085 | |||
| mean ± SD | 62.6 ± 8.5 years | 60.0 ± 6.3 years | 65.2 ± 9.7 years | |
| median [range] | 62.5 [44–80] | 61 [45–70] | 65 [44–80] | |
| KPS at EBRT; | .192 | |||
| mean ± SD | 85 ± 9 | 83 ± 10 | 87 ± 8 | |
| median [range] | 90 [60–100] | 80 [60–100] | 90 [70–100] | |
| Underlying liver disease; N (column %) | .490 | |||
| None | 3 (9%) | 2 (12%) | 1 (6%) | |
| Cirrhosis, unknown etiology | 7 (21%) | 2 (12%) | 5 (29%) | |
| Hepatitis B and/or C | 19 (56%) | 10 (65%) | 8 (47%) | |
| Alcoholic cirrhosis | 1 (3%) | 0 (0%) | 1 (6%) | |
| NASH | 3 (9%) | 2 (12%) | 1 (6%) | |
| Alpha 1 antitrypsin deficiency | 1 (3%) | 0 (0%) | 1 (6%) | |
| Childs Pugh Score; N (column %) | .098 | |||
| 5A | 21 (62%) | 11 (65%) | 10 (59%) | |
| 6A | 10 (29%) | 3 (18%) | 7 (41%) | |
| 7B | 3 (9%) | 3 (18%) | 0 (0%) | |
| AFP in IU/mL; | .050 | |||
| mean ± SD, | 6350 ± 26517 | 11670 ± 36624 | 697 ± 1801 | |
| median [range] | 48 [1.7–152352] | 503 [1.7–152342] | 14 [2.7–6691] | |
| Location of PVTT; N (column %) | .460 | |||
| Main PV | 12 (35%) | 7 (41%) | 5 (29%) | |
| R proximal PV | 9 (26%) | 3 (18%) | 6 (35%) | |
| L proximal PV | 7 (21%) | 5 (29%) | 2 (12%) | |
| R segmental PV | 4 (12%) | 1 (6%) | 3 (18%) | |
| L segmental PV | 2 (6%) | 1 (6%) | 1 (6%) | |
| T-stage; N (column %) | .194 | |||
| T1 | 2 (6%) | 1 (6%) | 1 (6%) | |
| T2 | 6 (18%) | 1 (6%) | 5 (29%) | |
| T3 | 26 (76%) | 145(88%) | 11 (65%) | |
| N-stage; N (column %) | .146 | |||
| N0 | 29 (85%) | 13 (76%) | 16 (94%) | |
| N1 | 5 (15%) | 4 (24%) | 1 (6%) | |
| M-stage; N (column %) | .310 | |||
| M0 | 33 (97%) | 17 (100%) | 16 (94%) | |
| M1 | 1 (3%) | 0 (0%) | 1 (6%) | |
| Prior treatment*; N (column %) | .473 | |||
| None | 12 (35%) | 7 (41%) | 5 (29%) | |
| TACE | 16 (47%) | 6 (35%) | 10 (59%) | |
| RFA | 4 (12%) | 2 (12%) | 2 (12%) | |
| Systemic therapy | 15 (44%) | 9 (53%) | 6 (35%) | |
| Radiation modality; N (column %) | .028 | |||
| 3DCRT | 3 (9%) | 2 (12%) | 1 (6%) | |
| IMRT | 22 (65%) | 14 (82%) | 8 (47%) | |
| PBR | 6 (18%) | 1 (6%) | 5 (29%) | |
| SBRT | 3 (9%) | 0 (0%) | 3 (18%) | |
| Gross tumor volume target in cubic centimeters; | .003 | |||
| mean ± SD, | 274 ± 254 | 357 ± 251 | 187 ± 235 | |
| median [range] | 189 [131–339] | 261 [188–456] | 137 [42–192] | |
| Radiation dose in Gy; | <0.001 | |||
| mean ± SD, | 55 ± 9 | 48.0 ± 4.9 | 62.1 ± 7.4 | |
| median [range] | 55 [40–75] | 45 [40–57.5] | 62.5 [45–75] | |
| Number of fractions; | .074 | |||
| mean ± SD, | 19 ± 8 | 22 ± 6 | 17 ± 8 | |
| median [range] | 17.5 [3–30] | 25 [10–28] | 15 [3–30] | |
| BED in Gy; | <0.001 | |||
| mean ± SD | 77 ± 25 | 59 ± 7 | 94 ± 24 | |
| median [range] | 75 [47–180] | 59 [47–75] | 86 [76–180] | |
| Breath-hold technique; N (column %) | 1.00 | |||
| Yes | 16 (47%) | 8 (47%) | 8 (47%) | |
| No | 18 (53%) | 9 (53%) | 9 (53%) | |
| CT-on rails image guidance; N (column %) | .724 | |||
| Yes | 12 (38%) | 6 (35%) | 7 (41%) | |
| No | 21 (62%) | 11 (65%) | 10 (59%) | |
| Concurrent chemotherapy; N (column %) | .015 | |||
| None | 22 (65%) | 7 (41%) | 15 (88%) | |
| Nexavar | 8 (24%) | 7 (41%) | 1 (6%) | |
| Xeloda | 4 (12%) | 3 (18%) | 1 (6%) |
BED = biologically effective dose; Gy = Gray; EBRT = external beam radiotherapy; SD = standard deviation; KPS = Karnofsky Performance Status; NASH = non-alcoholic hepatic steatosis; AFP = alpha-fetoprotein; IU/mL = international units per milliliter; PVTT = portal vein tumor thrombus; PV = portal vein; R = right; L = left; TACE = transarterial chemoembolization; RFA = radiofrequency ablation; 3DCRT = 3D conformal radiotherapy; IMRT = intensity-modulate radiotherapy; PBR = proton beam radiotherapy; SBRT = stereotactic body radiotherapy.
As some patients received more than one type of prior therapy, percentages do not add up to 100%.
Local control and patterns of recurrence
Local control at one year was 57.1%. Local recurrence was defined as an in-field or marginal failure. At the time of analysis, eight patients had developed a local recurrence. Six patients had tumor recurrence within the PTV volume, and the remaining two patients developed marginal recurrences. Of all the factors evaluated, only BED >75 Gy was associated with improved local control on univariate analysis (HR [95% CI] 0.21 [0.04–0.95]; p = 0.043) (Table 3).
Table 3.
Univariate analysis of local control and overall survival by demographic, disease and treatment characteristics of patients treated with external beam radiation for hepatocellular carcinoma portal vein tumor thrombus.
| Local Control |
Overall Survival |
|||
|---|---|---|---|---|
| HR [95% CI] | Univariate P-value* | HR [95% CI] | Univariate P-value* | |
| Gender | ||||
| Men | Reference | Reference | ||
| Women | 0.53 [0.03–3.06] | .530 | 0.84 [0.33–2.58 | .740 |
| Age at EBRT start in years | ||||
| ≤63 | Reference | Reference | ||
| >63 | 3.20 [0.74–21.86] | .125 | 1.45 [0.63–3.40] | .381 |
| KPS at EBRT | ||||
| >80 | Reference | Reference | ||
| ≤80 | 1.29 [0.26–5.31] | .734 | 0.48 [0.21–1.11] | .083 |
| Underlying liver disease | ||||
| No | Reference | Reference | ||
| Yes | 0.55 [0.09–10.63] | .651 | 0.80 [0.22–5.06] | .769 |
| Childs Class | ||||
| A | Reference | Reference | ||
| B | N/A€ | .303 | 0.70 [0.20–4.44] | .651 |
| AFP in IU/mL | ||||
| ≤1500 | Reference | Reference | ||
| >1500 | 1.53 [0.22–7.19] | .623 | 1.89 [0.65–5.01] | .228 |
| Location of PVTT | ||||
| Unilateral | Reference | Reference | ||
| Bilateral | 1.06 [0.22–4.33] | .937 | 1.06 [0.45–2.64] | .903 |
| T-stage | ||||
| T1 | Reference | Reference | ||
| T2 | 0.21 [0.01–5.47] | .299 | 0.87 [0.05–5.45] | .895 |
| T3 | 0.33 [0.05–6.47] | .379 | 1.19 [0.07–6.04] | .871 |
| N-stage | ||||
| N0 | Reference | Reference | ||
| N1 | 3.39 [0.47–17.48] | .196 | 0.34 [0.12–1.08] | .067 |
| Prior treatment | ||||
| No | Reference | Reference | ||
| Yes | 0.90 [0.22–4.42] | .891 | 1.23 [0.52–3.24] | .643 |
| Gross tumor volume target in cubic centimeters; | ||||
| ≤190 | Reference | Reference | ||
| >190 | 2.99 [0.71–15.0] | .133 | 1.67 [0.73–3.90] | .225 |
| Radiation modality | ||||
| 3DCRT | Reference | Reference | ||
| IMRT | N/A£ | 1.49 [0.42–9.48] | .578 | |
| PBR | N/A£ | 0.49 [0.08–3.76] | .452 | |
| SBRT | N/A£ | 0.45 [0.02–4.75] | .507 | |
| Radiation dose | ||||
| ≤55 Gy | Reference | Reference | ||
| >55 Gy | 0.43 [0.10–1.88] | .248 | 0.42 [0.17–1.01] | .053 |
| BED | ||||
| ≤75 Gy | Reference | Reference | ||
| >75 Gy | 0.21 [0.04–0.95] | .043 | 0.26 [0.10–0.63] | .003 |
| Breath-hold technique | ||||
| Yes | Reference | Reference | ||
| No | 1.31 [0.31–5.54] | .706 | 0.88 [0.38–2.09] | .764 |
| CT-on rails image guidance | ||||
| Yes | Reference | Reference | ||
| No | 0.51 [0.12–2.16] | .344 | 0.87 [0.37–2.18] | .754 |
| Concurrent chemotherapy | ||||
| Yes | Reference | Reference | ||
| No | 0.66 [0.16–3.24] | .576 | 0.70 [0.30–1.76] | .433 |
EBRT = external beam radiotherapy; KPS = Karnofsky Performance Status; AFP = alpha-fetoprotein; IU/mL = international units per milliliter; PVTT = portal vein tumor thrombus; 3DCRT = 3D conformal radiotherapy; IMRT = intensity-modulate radiotherapy; PBR = proton beam radiotherapy; SBRT = stereotactic body radiotherapy; BED = biologically effective dose; Gy = Gray.
Univariate P-value is from the Cox proportional hazards regression model.
Only three patients had Child-Pugh B disease and none of them experienced local failure.
None of the 9 patients in the 3DCRT group experienced local failure, and none of the 3 patients in the SBRT group experienced local failure.
Liver control at one year was 36.4%. At the time of analysis, 17 patients developed new metastatic lesions in the liver. Distant control at one year was 55.2%. Extrahepatic metastases ultimately developed in 18 patients, including nine patients with lung metastases, four patients with distant nodal metastases, two patients each with bone and adrenal metastases and one patient with disseminated peritoneal metastases. One patient had lung metastasis at the time of radiation, and this patient was excluded from this analysis.
Survival outcomes and causes of death
The median overall survival time was 13.4 months, and the 1-year OS was 57.4% for the entire cohort. Of the 23 patients who died, 17 died of decompensated liver failure, one died to complications related to bleeding esophageal varices, one died of acute respiratory distress syndrome, one died after a pathologic hip fracture, and the remaining three died of unknown causes.
Prognostic factors for overall survival
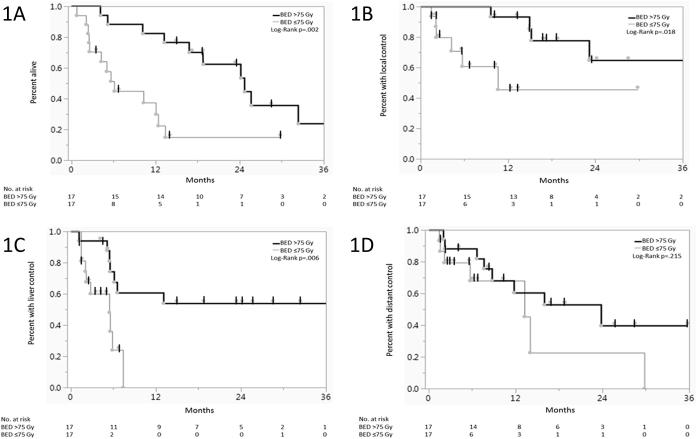
Of all the potential variables examined (Table 3), only Karnofsky Performance Status (KPS) (≤80 vs >80), N-stage (N0 vs N1), radiation dose (≤55 Gy vs >55 Gy) and BED (≤75 Gy vs >75 Gy) were significant. Patients who received a BED >75 Gy had significantly better median survival (24.7mo vs 6.1mo) and 1-year OS (76.5% vs 30.0%) when compared with those who received a BED ≤75 Gy (Log-Rank p = 0.002) (Fig. 1). Patients who received a BED >75 Gy likewise had improved local control and liver control but not distant control (Fig. 1, respectively). By multivariate analysis (Table 4), BED >75 Gy was an independent predictive factor for overall survival (p = 0.015). BED was not predictive for overall survival when analyzed as a continuous variable, and nominal radiation dose was also not predictive for overall survival when analyzed as either a continuous variable or divided into groups of >55 Gy vs ≤55 Gy (the median dose delivered).
Fig. 1.
Effect of Biologically Effective Dose (BED) on Overall Survival (1A), Local Control (1B), Liver Control (1C) and Distant Control (1D) from the time of initiation of radiation.
Table 4.
Multivariate analysis of variables associated with overall survival in patients treated with external beam radiation for hepatocellular carcinoma portal vein tumor thrombus.
| HR | 95% CI | P-value | |
|---|---|---|---|
| KPS at EBRT | .087 | ||
| >80 | Reference | Reference | |
| ≤80 | 2.29 | 0.89–6.14 | |
| N-stage | .295 | ||
| N0 | Reference | Reference | |
| N1 | 2.04 | 0.52–7.24 | |
| Radiation dose as a continuous variable* | 1.04 | 0.96–1.13 | .393 |
| Radiation dose | .133 | ||
| ≤55 Gy | Reference | Reference | |
| >55 Gy | 0.39 | 0.10–1.31 | |
| BED as a continuous variable* | 1.03 | 0.99–1.06 | .058 |
| BED | .015 | ||
| ≤75 Gy | Reference | Reference | |
| >75 Gy | 0.10 | 0.01–0.66 |
EBRT = external beam radiotherapy; KPS = Karnofsky Performance Status; BED = biologically effective dose; Gy = Gray.
HR listed is per Gy.
Toxicity
Two patients developed grade 3 upper GI bleeding outside of the treatment field thought to be related to pre-existing portal hypertension. One patient developed acute grade 2 nausea. Otherwise, 26 patients (78.8%) of patients developed at least one acute grade 1 toxicity during treatment including fatigue, nausea, dermatitis and diarrhea.
All but three patients were able to complete RT as planned. One patient’s RT was stopped after 22 of a planned 25 fractions of 1.8 Gy each due to rising bilirubin, transaminases and alkaline phosphatase. A second patient’s RT was stopped after 14 of a planned 25 fractions of 3 Gy each due to large-volume bleeding from esophageal varices. Another patient’s RT was stopped after 23 of a planned 25 fractions of 2.5 Gy each because daily CT image guidance revealed his stomach was closer to the high dose region than that initially seen on planning CT. So that the dose constraint of total maximum dose <54 Gy to the stomach could be respected, the last two fractions were omitted.
The median [IQR] mean liver minus GTV dose for this cohort was 22.4 Gy [18.9–24.7 Gy]. There were no cases of suspected radiation-induced liver disease (RILD), as patients who died of liver failure all had progression of intrahepatic disease.
Discussion
The results of this retrospective study suggest that definitive RT is a safe and well-tolerated approach for patients with HCC PVTT in the United States. LC at 1 year was approximately 74% in this cohort, and 1-year OS was approximately 59% with the majority of patients experiencing progression either elsewhere in the liver or at distant sites. Though we did not identify a significant relationship between total radiation dose and OS, when both total dose and dose per fraction were taken into account, BED >75 Gy was significantly associated with improved OS.
There have been no published reports to date outlining outcomes of Western patients with HCC PVTT who received radiation therapy. The majority of previously published reports come from Asian countries where the incidence of HCC is higher [23]. The LC and OS survival of patients in our cohort were much better than the 20–45% 1-year OS rates previously reported using either 3DCRT alone [12], [14], [15] or sequentially after TACE [13]. In contrast to prior studies [14], we saw no significant association with Child Pugh class and survival, but this may be due to the relatively few patients with Child Pugh class B liver function included in our cohort. The largest retrospective review of 326 patients with HCC PVTT is from Taiwan reported a median survival of 13.3 months. Pre-treatment performance status and radiation dose ≥50 Gy were most strongly associated with improved survival outcomes [16]. In contrast, we saw no significant relationship between performance status and OS. Furthermore, although we saw no difference in OS by total delivered radiation dose, this may be due to the large amount of heterogeneity in the dose-fractionation schedules used. When we analyzed survival by BED, we did see a significant dose-response relationship with patients receiving BED >75 Gy experiencing significantly better OS. A subset of our patients received concurrent sorafenib with radiation, but this did not seem to impact outcomes. Although concurrent sorafenib has not been studied specifically in HCC patients with PVTT, data regarding concurrent sorafenib with radiation are mixed. Response rates appear promising, but there have been serious toxicities reported, including fatal liver failure [27], [28]. Since the publication of these studies, our group has proceeded with caution regarding the use of concurrent sorafenib with liver-directed radiation.
We did not identify any difference in LC or OS between patients treated with 3DCRT, IMRT, PBR or SBRT. Other groups have reported encouraging survival and control rates using advanced radiation techniques such as PBR [18], [19] hypofractionated PBR [20] or SBRT [21] for patients with HCC and PVTT. In a single-arm phase II study, patients treated hyprofractionated PBR to 67.5 Gy in 15 fractions had a reported 63.2% 2-year OS [20]. A retrospective analysis of patients treated with SBRT to 36 Gy in 6 fractions reported a 1-year OS similar to ours at 50.3% [22]. We reported low rates of serious toxicity among patients in our cohort and no suspected cases of RILD. At our center, we limit the mean liver-minus-GTV volume to <28 Gy for 1.8-2 Gy per fraction and to <24 Gy for >4 Gy per fraction. We do this by minimizing PTV expansions by using conformal radiation techniques with daily CT-based IGRT and a breath hold technique for patients treated with higher doses [26], [29].
Strengths of this study include being the first to confirm in a Western population the findings of a prior retrospective study suggesting a potential dose–response relationship with overall survival [16]; though our data suggest BED, and not total dose delivered, may be the primary driving factor. BED has also been shown to be a favorable prognostic factor in the definitive treatment of intrahepatic cholangiocarcinomas, where BED >80.5 Gy was suggested to confer a survival advantage [30].
This study is not without the limitations inherent to any single-institution retrospective review. We cannot fully account for subtle selection biases that may have favored patients treated with a higher BED. The fact that patients who received a higher BED also had a smaller gross tumor volume treated supports this; however, the gross tumor volume size was not significantly associated with either local control or overall survival, so it is likely dose escalation still matters in this population. The fact that this was a heavily pretreated population reflects the reality of referral patterns in that patients are often not referred for consideration of radiation until they have failed or progressed through one or more other therapies. Similarly, the radiation modality used is also heterogeneous in this group and may limit conclusions that can be drawn. However, we also feel this represents the reality of our practice where the radiation modality is chosen that can offer the best chance at dose escalation based on the individual patient’s tumor location, size and underlying liver function and other comorbidities. Finally, toxicity data may be incomplete as recorded from the medical record, and the relatively short follow up period for some patients makes it possible that long-term toxicities from this treatment were not fully captured.
In conclusion, our data suggest that RT should be considered for well-selected patients with HCC and PVTT for the purpose of prolonging the time to worsening obstruction and liver failure. When feasible, dose-escalation should be considered with a target BED of >75 Gy if liver, duodenal, stomach and other critical organ dose constraints can be safely met.
Conflicts of interest
None.
Funding source
None.
References
- 1.Minagawa M., Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirisi M., Avellini C., Fabris C. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397–400. doi: 10.1007/s004320050189. [DOI] [PubMed] [Google Scholar]
- 3.Okuda K., Ohtsuki T., Obata H. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Bustamante J., Castells A. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 5.Anon. National Comprehensive Cancer Network Guidelines Version 2.2016: Hepatocellular Carcinoma.
- 6.Anon. European Association for the Study of the Liver Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma.
- 7.Park H.C., Yu J.I., Cheng J.C.-H. Consensus for radiotherapy in hepatocellular carcinoma from the 5th Asia-pacific primary liver cancer expert meeting (APPLE): current practice and future clinical trials. Liver Cancer. 2014;2016(5):162–174. doi: 10.1159/000367766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson L.A., Normolle D., Balter J.M. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J.C-H., Liu H-S., Wu J.K. Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2005;62:1150–1156. doi: 10.1016/j.ijrobp.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Kim T.H., Kim D.Y., Park J.-W. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225–231. doi: 10.1016/j.ijrobp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Bae B.K., Kim J.-C. The response of thrombosis in the portal vein or hepatic vein in hepatocellular carcinoma to radiation therapy. Radiat Oncol J. 2016 doi: 10.3857/roj.2016.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S.H., Lin Y.M., Chuang V.P. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 13.Ishikura S., Ogino T., Furuse J. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa K., Yamashita H., Shiraishi K. Radiation therapy for portal venous invasion by hepatocellular carcinoma. World J Gastroenterol. 2005;11:7237–7241. doi: 10.3748/wjg.v11.i46.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Z.-C., Fan J., Tang Z.-Y. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y.-J., Hsu H.-C., Wang C.-Y. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 17.Wang K., Guo W.X., Chen M.S. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: a large-scale, multicenter, propensity matching score analysis. Medicine. 2016;95:e3015. doi: 10.1097/MD.0000000000003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata M., Tokuuye K., Sugahara S. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2005;104:794–801. doi: 10.1002/cncr.21237. [DOI] [PubMed] [Google Scholar]
- 19.Sugahara S., Nakayama H., Fukuda K. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 20.Hong T.S., Wo J.Y., Yeap B.Y. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuo Y., Yoshida K., Nishimura H. Efficacy of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis/inferior vena cava tumor thrombosis: evaluation by comparison with conventional three-dimensional conformal radiotherapy. J Radiat Res. 2016 doi: 10.1093/jrr/rrw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi M., Zhang L., Zhao L. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu R.X., Seto W.-K., Lai C.-L. Epidemiology of hepatocellular carcinoma in the Asia-pacific region. Gut Liver. 2016;10:332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoller H., Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism. 2016;65:1151–1160. doi: 10.1016/j.metabol.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Peng Y., Vedam S., Chang J.Y. Implementation of feedback-guided voluntary breath-hold gating for cone beam CT-based stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:909–917. doi: 10.1016/j.ijrobp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Crane C.H., Koay E.J. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer. 2016;122:1974–1986. doi: 10.1002/cncr.29878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S.-W., Lin L.-C., Kuo Y.-C. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041–1047. doi: 10.1016/j.ijrobp.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Brade A.M., Ng S., Brierley J. Phase 1 trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2016;94:580–587. doi: 10.1016/j.ijrobp.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 29.Cheng J.C.-H., Wu J.-K., Huang C.-M. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–162. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 30.Tao R., Krishnan S., Bhosale P.R. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]