Fig. 1. Schematic illustration of electrodeposition behaviors and synergistic effect at the molecular level.
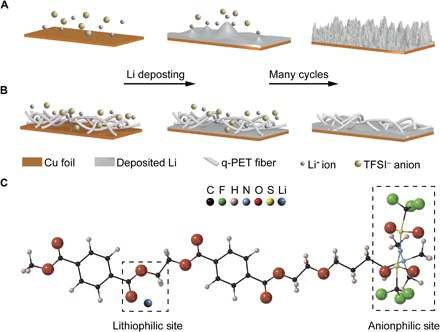
(A) Lithium deposition on a routine copper foil. Li-ion flux is more concentrated on the dendrite tip, and the concentration of anions drops near the anode surface, resulting in self-enhanced dendrite growth on repeated cycling. (B) Lithium deposition on q-PET interlayer/Cu. q-PET can attract large quantities of Li ions and bis(trifluoromethanesulfonyl)imide (TFSI) anions from its polar functional groups. Uniform ion distribution at the anode surface promotes smooth deposition. (C) Sketch of the structure of q-PET. Dendrite-free Li deposition is facilitated via rationally engineered binding toward both Li cation and TFSI anion.
