Abstract
Background
Pertuzumab disrupts heterodimerization between human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR), HER3, and HER4. Thus, pertuzumab could result in adverse events similar to those observed with EGFR antagonists, such as diarrhea. We report the incidence and severity of diarrhea observed with pertuzumab in the CLEOPATRA, NeoSphere, and TRYPHAENA studies.
Patients and methods
Patients (n = 1443) had metastatic [CLEOPATRA (n = 804)] or early-stage breast cancer [NeoSphere (n = 416) and TRYPHAENA (n = 223)]. The incidence and severity of diarrhea were analyzed by treatment received. The incidence of febrile neutropenia concurrent with diarrhea and the effect of pre-existing gastrointestinal comorbidities were also evaluated. Subgroup analyses were carried out using CLEOPATRA data.
Results
The incidence of all-grade diarrhea across studies was generally greater for pertuzumab-based treatment, ranging from 28% to 72% (grade 1, 21%–54%; grade 2, 8%–37%; grade 3, 0%–12%; grade 4, 0%). Incidence was highest during the first pertuzumab-containing cycle, decreasing with subsequent cycles. Dose delays or discontinuations due to diarrhea were infrequent, ranging from 0% to 8%. Among pertuzumab-treated patients with diarrhea, 47%–67% received pharmacological intervention, most commonly with loperamide. Overlap between diarrhea and febrile neutropenia was uncommon, ranging from 0% to 11%. No relationship was observed between pre-existing gastrointestinal comorbidities and diarrhea. In CLEOPATRA, patients ≥65 years treated with pertuzumab had a higher incidence of grade 3 diarrhea than patients <65 years (19% versus 8%). All-grade diarrhea occurred at greater frequency among pertuzumab-treated Asian versus white patients with metastatic breast cancer (74% versus 63%); the corresponding rates in the control arm were 53% and 45%, respectively.
Conclusions
In both the metastatic and early-stage breast cancer settings, diarrhea was common but manageable for all pertuzumab-containing regimens. Diarrheal episodes were mainly low grade and occurred most often during the first treatment cycle. Diarrheal-related drug delays or discontinuations were uncommon.
ClinicalTrials.gov identifiers
NCT00567190 (CLEOPATRA), NCT00545688 (NeoSphere), NCT00976989 (TRYPHAENA).
Keywords: pertuzumab, diarrhea, breast cancer, docetaxel, HER2, EGFR
Introduction
Pertuzumab, in combination with trastuzumab and docetaxel, is an effective treatment option for patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer in both the metastatic and neoadjuvant settings. Pertuzumab is a monoclonal antibody that binds to HER2 at extracellular subdomain II and impedes ligand-dependent dimerization with other HER receptors, including epidermal growth factor receptor (EGFR), HER3, and HER4 [1], thereby inhibiting proliferative signaling in HER2-positive tumor cells. Because it prevents HER2 from heterodimerizing with EGFR (thus blocking downstream signaling cascades triggered by this partnership) [2], pertuzumab may lead to adverse events (AEs) associated with EGFR antagonists, such as diarrhea. HER2 and EGFR are expressed on intestinal epithelial cell membranes and act in concert to negatively regulate chloride secretion via the phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) pathways [3]. As such, excess chloride secretion is postulated to underlie the secretory diarrhea observed in up to 87% of patients treated with EGFR tyrosine-kinase inhibitors (TKIs) [3, 4]. This mechanism differs from that observed in chemotherapy-induced diarrhea, which is secondary to mucositis of the gastrointestinal tract [5].
This exploratory analysis was designed to quantify the incidence and severity of diarrhea with pertuzumab-containing regimens, the impact of diarrhea on treatment course and management, the occurrence of diarrhea concurrent with febrile neutropenia, and whether particular patient subgroups defined by age and race are differentially affected. Here, we summarize data from the safety populations of three trials examining pertuzumab-containing regimens: CLEOPATRA, NeoSphere, and TRYPHAENA.
Methods
Study designs
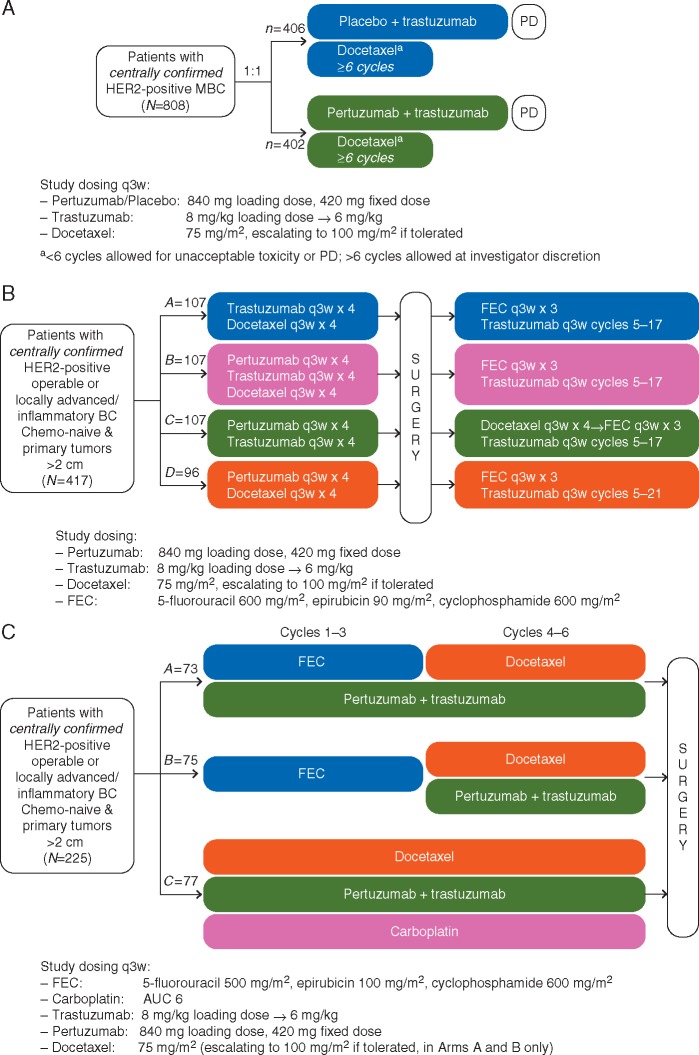
The phase III CLEOPATRA study (NCT00567190) evaluated trastuzumab and docetaxel plus either pertuzumab or placebo (P + H + D and placebo + H + D, respectively) in patients with previously untreated HER2-positive metastatic breast cancer (Figure 1A) [6, 7]. The phase II studies NeoSphere (NCT00545688) (Figure 1B) [8] and TRYPHAENA (NCT00976989) (Figure 1C) [9] evaluated pertuzumab in the neoadjuvant setting only. In NeoSphere, patients were randomized to receive trastuzumab + docetaxel (H + D; Arm A), pertuzumab + trastuzumab + docetaxel (P + H + D; Arm B), pertuzumab + trastuzumab (P + H; Arm C), or pertuzumab + docetaxel (P + D; Arm D); four cycles of these regimens were administered before surgery (chemotherapy and trastuzumab, but not pertuzumab, were administered post-surgery). All three neoadjuvant treatment arms in TRYPHAENA contained pertuzumab: 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) + P + H x3 cycles → P + H + D x3 (Arm A); FEC x3 → P + H + D x3, in which the first pertuzumab-containing cycle was cycle 4 (Arm B); and docetaxel + carboplatin + trastuzumab + pertuzumab (TCH + P) x6 (Arm C). The designs of these three studies have been described [6–9]. These studies were approved by the institutional review boards at participating centers and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Figure 1.
Study designs of (A) CLEOPATRA, (B) NeoSphere, and (C) TRYPHAENA. (A) In CLEOPATRA [6, 7], patients with HER2-positive MBC were randomized to receive first-line trastuzumab and docetaxel plus pertuzumab or placebo until unacceptable toxicity or disease progression. Docetaxel treatment was recommended for a minimum of six cycles, after which patients were permitted to continue to receive HER2-targeted therapies. (B) In NeoSphere [8], patients with HER2-positive early-stage breast cancer were randomized to one of four neoadjuvant treatment regimens: trastuzumab + docetaxel (Arm A), pertuzumab + trastuzumab + docetaxel (Arm B), pertuzumab + trastuzumab (Arm C), or pertuzumab + docetaxel (Arm D) for four cycles. While adjuvant therapy regimens were also designated in NeoSphere, these regimens did not contain pertuzumab and were not included in the present analysis. (C) Similar to NeoSphere, TRYPHAENA [9] evaluated pertuzumab in the neoadjuvant setting only. Patients were randomized to receive six cycles of one of the following: 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) + pertuzumab + trastuzumab for three cycles, followed by pertuzumab + trastuzumab + docetaxel for three cycles (Arm A); FEC for three cycles, followed by pertuzumab + trastuzumab + docetaxel for three cycles, in which the first pertuzumab-containing cycle was cycle 4 (Arm B); or docetaxel + carboplatin + trastuzumab + pertuzumab for three cycles (Arm C). The sample sizes for the intent-to-treat populations are shown. BC, breast cancer; FEC, 5-fluorouracil, epirubicin, cyclophosphamide; HER2, human epidermal growth factor receptor 2; MBC, metastatic breast cancer; PD, progression of disease; q3w, every 3 weeks.
Treatment
Treatment was administered intravenously as indicated in each study (Figure 1) at the following dosages: pertuzumab, 840 mg loading dose followed by a 420 mg fixed dose every 3 weeks (q3w); docetaxel, 75 mg/m2 q3w; and trastuzumab, 8 mg/kg loading dose followed by 6 mg/kg q3w. Neoadjuvant FEC was administered q3w in TRYPHAENA Arms A and B at 500 mg/m2 (5-fluorouracil), 100 mg/m2 (epirubicin), and 600 mg/m2 (cyclophosphamide); patients in TRYPHAENA Arm C received carboplatin at a target area under the concentration–time curve (AUC) of 6. Study drugs were administered per protocol guidance or until disease progression, unmanageable toxicity, or physician decision.
Pertuzumab and trastuzumab doses could be interrupted or delayed to manage AEs in all studies, but dose reductions were not permitted. If tolerated, docetaxel could be escalated to 100 mg/m2 in all studies except for Arm C of TRYPHAENA; to manage toxicity, docetaxel could be reduced to 55 mg/m2 in CLEOPATRA or 60 mg/m2 in NeoSphere and TRYPHAENA.
Data analysis
Data from the safety populations (i.e. randomized patients who received at least one full or partial dose of any study medication) of each study were analyzed. Patients randomized to the placebo arm of CLEOPATRA who crossed over to pertuzumab were censored the day before crossover; all data up to the censored date were analyzed (data cutoff: February 2014). All patients from the neoadjuvant phase (cycles 1–4) of NeoSphere who had at least one safety assessment carried out at baseline were analyzed (data cutoff: October 2014). All patients from the neoadjuvant phase (cycles 1–6) of TRYPHAENA were analyzed (data cutoff: July 2012). AEs in each study were monitored continuously and graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Descriptive analyses were carried out to summarize diarrhea-related parameters by treatment arm, including the number of treatment cycles and the administration of antidiarrheal medications. The incidence of diarrhea was summarized at the patient- and episode-level by grade and treatment cycle. For the patient-level analysis, only the most severe diarrheal episode or the diarrheal episode with the strongest causal relationship to study drug (if the patient had repeated episodes of diarrhea) was counted.
Incidences of febrile neutropenia and pre-existing gastrointestinal disorders (irritable bowel syndrome, colitis, or Crohn’s disease) in patients with any grade of diarrhea were evaluated. Concurrent diarrhea and febrile neutropenia was defined as any grade of either event that overlapped for at least 1 day.
Subgroup analyses were carried out by age (<65 versus ≥65 years) and race (white versus Asian, as these were the only groups with sufficient numbers for analysis) in CLEOPATRA only. The numbers of patients in each treatment arm in the early-stage breast cancer studies were too small to conduct meaningful subgroup analyses.
Results
Patients
A total of 1443 patients were included in this exploratory analysis (supplementary Figure S1, available at Annals of Oncology online). Of the 396 patients randomized to placebo + H + D in CLEOPATRA, 45 (11.4%) crossed over to P + H + D; in the present analysis, these patients were censored the day before crossing over. Baseline demographics and disease characteristics in the intent-to-treat populations of CLEOPATRA, NeoSphere, and TRYPHAENA have been published [6, 8, 9].
Incidence, severity, and management of diarrhea
The incidence and management of diarrhea in patients with metastatic or early-stage breast cancer are shown in Tables 1 and 2, respectively. Across all treatment arms, the median number of diarrheal episodes per patient was 2 in CLEOPATRA and 1 in NeoSphere and TRYPHAENA. The incidence of at least one episode of any-grade diarrhea in patients receiving pertuzumab-based treatment ranged from 28% in the chemotherapy-free arm of NeoSphere (P + H) to 72% in the TCH + P arm of TRYPHAENA. In NeoSphere, diarrhea occurred more frequently among patients receiving both pertuzumab and docetaxel (46% for P + H + D and 54% for P + D versus 28% for P + H and 34% for the non-pertuzumab-containing H + D arm). In CLEOPATRA, there was an increased diarrheal incidence of ∼19% points in the pertuzumab-containing arm relative to the control arm (68% for P + H + D versus 49% for placebo + H + D).
Table 1.
Incidence and management of diarrheal episodes in patients with HER2-positive metastatic breast cancer
| CLEOPATRAa | ||
|---|---|---|
| P + H + D | Placebo + H + D | |
| n = 408 | n = 396 | |
| Median number of pertuzumab or placebo cycles (range) | 24 (1–96) | 15 (1–92) |
| Median number of docetaxel cycles (range) | 8 (1–52) | 8 (1–42) |
| Patients with ≥1 episode of any-grade diarrhea, n (%) | 279 (68) | 193 (49) |
| Severityb | ||
| Grade 1 | 220 (54) | 150 (38) |
| Grade 2 | 143 (35) | 87 (22) |
| Grade 3 | 38 (9) | 19 (5) |
| Median number of diarrheal episodes per patient (range) | 2 (1–46) | 2 (1–20) |
| Median time to first diarrheal episode, days (IQR) | 8 (4–44) | 23 (6–68) |
| Patients with diarrhea leading to discontinuation of any study drug, n (%) | 8 (2) | 2 (0.5) |
| Patients with diarrhea leading to dose interruption/modification (ie, dose delay, not reduction), n (%) | 25 (6) | 7 (2) |
| Patients treated with ≥1 antidiarrheal medication, n (%)c | 164 (59) | 79 (41) |
| Patients treated with loperamide, n (%)c | 158 (57) | 77 (40) |
Safety population (as described in the Methods section).
One patient in the control arm experienced grade 4 diarrhea.
Percentages were calculated using the number of patients with ≥1 episode of diarrhea as the denominator.
IQR, interquartile range; P + H + D, pertuzumab + trastuzumab + docetaxel; placebo + H + D, placebo + trastuzumab + docetaxel.
Table 2.
Incidence and management of diarrheal episodes during neoadjuvant treatment in patients with HER2-positive early-stage breast cancer
|
NeoSpherea |
TRYPHAENAa |
||||||
|---|---|---|---|---|---|---|---|
| H + D x4 | P + H + D x4 | P + H x4 | P + D x4 | FEC + P + H x3 → P + H + D x3 | FEC x3 → P + H + D x3 | TCH + P x6 | |
| (Arm A) | (Arm B) | (Arm C) | (Arm D) | (Arm A) | (Arm B) | (Arm C) | |
| n = 107 | n = 107 | n = 108 | n = 94 | n = 72 | n = 75 | n = 76 | |
| Patients with ≥1 episode of any-grade diarrhea, n (%) | 36 (34) | 49 (46) | 30 (28) | 51 (54) | 44 (61) | 46 (61) | 55 (72) |
| Severityb | |||||||
| Grade 1 | 27 (25) | 39 (36) | 23 (21) | 36 (38) | 31 (43) | 29 (39) | 34 (45) |
| Grade 2 | 10 (9) | 17 (16) | 9 (8) | 22 (23) | 20 (28) | 17 (23) | 28 (37) |
| Grade 3 | 4 (4) | 6 (6) | 0 | 4 (4) | 3 (4) | 4 (5) | 9 (12) |
| Median number of diarrheal episodes per patient (range) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (1 –3) | (1 –6) | (1 –4) | (1 –4) | (1 –6) | (1 –4) | (1 –6) | |
| Median time to first diarrheal episode, days (IQR) | 6 | 7 | 5 | 6 | 7 | 69c | 6 |
| (3 –15) | (3 –22) | (2 –15) | (3 –16) | (4 –27) | (64 –81) | (3 –21) | |
| Patients with diarrhea leading to discontinuation of any study drug, n | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patients with diarrhea leading to dose interruption/ modification (i.e. dose delay, not reduction), n (%) | 1 (1) | 8 (7) | 0 | 4 (4) | 0 | 4 (5) | 3 (4) |
| Patients treated with ≥1 antidiarrheal medication, n (%)d | 13 (36) | 23 (47) | 20 (67) | 28 (55) | 23 (52) | 22 (48) | 31 (56) |
| Patients treated with loperamide, n (%)d | 11 (31) | 19 (39) | 18 (60) | 26 (51) | 23 (52) | 22 (48) | 31 (56) |
Safety populations (as described in the Methods section).
No patient in NeoSphere or TRYPHAENA experienced grade 4 or 5 diarrhea.
Cycle 4 was the first pertuzumab-containing cycle.
D, docetaxel; FEC, 5-fluorouracil, epirubicin, cyclophosphamide; H, trastuzumab; IQR, interquartile range; P, pertuzumab; TCH, docetaxel (T), carboplatin (C), trastuzumab (H).
Percentages were calculated using the number of patients with ≥1 episode of diarrhea as the denominator.
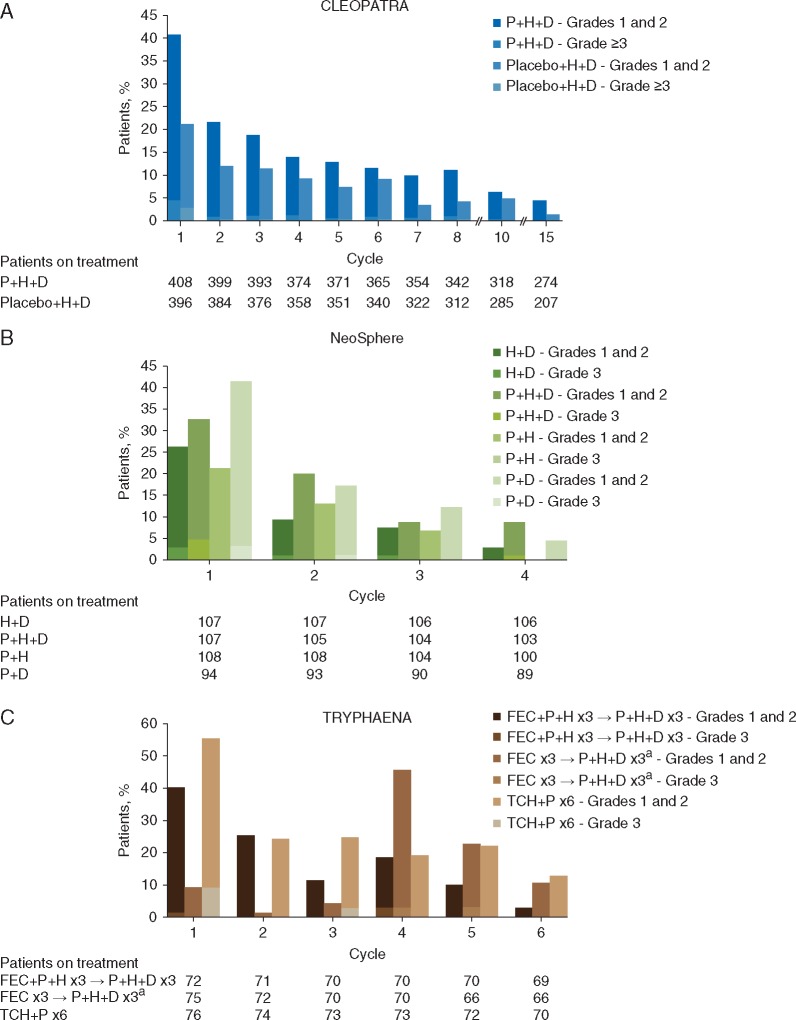
For the subset of patients in CLEOPATRA who continued targeted therapy after discontinuing docetaxel, diarrheal incidence by patient and by cycle before and after docetaxel discontinuation was analyzed. In this subgroup, 314 patients received P + H + D and 270 received placebo + H + D before docetaxel discontinuation, with an average of 0.32 and 0.25 diarrheal episodes per treatment cycle, respectively. Following docetaxel discontinuation, average diarrheal incidence fell to 0.11 and 0.09 episodes per cycle, respectively. Of note, a lower incidence of diarrhea was commonly observed during later therapy cycles, regardless of docetaxel treatment (Figure 2). The overall incidence of diarrheal episodes was consistently greatest during the first pertuzumab-containing cycle of each study [i.e. cycle 1 for all pertuzumab-containing regimens, except for TRYPHAENA Arm B (FEC x3 → P + H + D x3) when pertuzumab was first administered at cycle 4], and generally decreased during subsequent treatment cycles for all regimens.
Figure 2.
Incidence of diarrhea by treatment cycle in (A) CLEOPATRA, (B) NeoSphere, and (C) TRYPHAENA. Percentages were calculated using the number of patients receiving treatment per arm and per cycle as denominators. aCycle 4 was the first pertuzumab-containing cycle. D, docetaxel; FEC, 5-fluorouracil, epirubicin, cyclophosphamide; IQR, interquartile range; P, pertuzumab; H, trastuzumab; TCH, docetaxel (T), carboplatin (C), trastuzumab (H).
Across all studies, most diarrheal episodes for each regimen were grade 1 (range: 21%–54%) and grade 2 (range: 8%–37%). In CLEOPATRA, 9% of patients receiving pertuzumab experienced grade 3 diarrhea compared with 5% in the control arm; one patient administered placebo + H + D had grade 4 diarrhea. In NeoSphere, grade 3 diarrhea was observed in the docetaxel-containing arms (P + H + D, 6%; P + D, 4%; H + D, 4%), with no cases noted in the P + H arm. The highest incidence of grade 3 diarrhea across all study arms was 12% in the TCH + P arm of TRYPHAENA. No grade 4 diarrhea was observed in NeoSphere or TRYPHAENA.
Among patients with metastatic breast cancer, 2% in the pertuzumab arm of CLEOPATRA discontinued a study drug because of diarrhea compared with 0.5% in the control arm. No patient discontinued any study drug due to diarrheal episodes in the early-stage breast cancer studies (NeoSphere and TRYPHAENA). Dose delays due to diarrhea were uncommon, occurring in ≤8% of patients across all studies; these patients were subsequently maintained on pertuzumab. In CLEOPATRA, dose delays due to diarrhea occurred in 6% of patients receiving P + H + D and 2% of patients receiving placebo + H + D. In the early-stage breast cancer studies, dose delays due to diarrhea occurred in 0% (P + H arm in NeoSphere and FEC + P+H x3 → P + H + D x3 arm in TRYPHAENA) to 8% (P + H + D arm in NeoSphere) of patients receiving pertuzumab-based treatment compared with 1% of patients in the non-pertuzumab-containing treatment arm of NeoSphere.
Among patients with diarrhea, a greater proportion of patients across all pertuzumab-containing arms in the three studies received antidiarrheal treatment relative to the non-pertuzumab-containing arms. Fifty-nine percent of patients with diarrhea in the pertuzumab arm of CLEOPATRA received at least one antidiarrheal medication compared with 41% in the control arm. In NeoSphere, 47%–67% of patients with diarrhea in the pertuzumab-containing arms received antidiarrheal medication versus 36% in the non-pertuzumab arm. Loperamide was the most frequently prescribed medication and was administered to 31%–60% of patients who experienced diarrhea.
Diarrheal episodes (any grade) concurrent with febrile neutropenia (any grade)
Concurrent diarrhea and febrile neutropenia of any grade was uncommon. In CLEOPATRA, this occurred in 19 patients (5%) in the pertuzumab arm and six patients (2%) in the control arm. In NeoSphere, this occurred in five patients (5%) in the H + D arm, two patients (2%) in the P + H + D arm, no patients in the P + H arm, and four patients (4%) in the P + D arm. In TRYPHAENA, concurrent diarrhea and febrile neutropenia occurred in five patients (7%) in the FEC + P+H x3 → P + H + D x3 arm, two patients (3%) in the FEC x3 → P + H + D x3 arm, and eight patients (11%) in the TCH + P arm.
Diarrheal episodes (any grade) concurrent with gastrointestinal comorbidities
No relationship between pre-existing gastrointestinal comorbidities (irritable bowel syndrome, colitis, and Crohn’s disease) and diarrheal episodes was observed in any study. Less than 2% of patients presented with a pre-existing gastrointestinal comorbidity: 13 patients (1.6%) in CLEOPATRA, seven patients (1.7%) in NeoSphere, and two patients (0.9%) in TRYPHAENA, with similar incidence across treatment arms.
Diarrheal episodes by age in patients with metastatic breast cancer (CLEOPATRA)
Irrespective of treatment, patients <65 versus ≥65 years of age exhibited similar rates of all-grade diarrhea (supplementary Table S1, available at Annals of Oncology online). However, in the pertuzumab-containing arm, grade ≥3 diarrhea occurred more frequently among patients aged ≥65 years [19% (12/62)] than <65 years [8% (26/346)]. Elderly patients receiving pertuzumab also had higher rates of drug discontinuation [5% (3/62) versus 1% (5/346)] and dose delays [15% (9/62) versus 5% (16/346)] due to diarrhea than patients in the control arm. The incidence of concurrent diarrhea and febrile neutropenia did not increase with age. In the control arm, overlapping diarrhea and febrile neutropenia occurred in 0% of patients (0/64) aged ≥65 years and 2% of patients (6/332) aged <65 years. In the pertuzumab arm, the rate of concurrent diarrhea and febrile neutropenia was the same for patients aged ≥65 years [5% (3/62)] and <65 years [5% (16/346]).
Diarrheal episodes by race in metastatic breast cancer (CLEOPATRA)
White and Asian patients comprised the two most common racial subgroups in CLEOPATRA. Compared with white patients, Asian patients appeared to have a greater incidence of all-grade diarrhea and grade ≥3 diarrhea, regardless of treatment (supplementary Table S2, available at Annals of Oncology online). However, dose delays due to diarrhea were more frequent in white patients [8% (2/250)] than in Asian patients [4% (5/128)]. In the P + H + D treatment arm, Asian patients also appeared to have a higher incidence of overlapping diarrhea and febrile neutropenia than white patients [9% (11/128) versus 3% (8/250)]. Relative to the pertuzumab arm, concurrent diarrhea and febrile neutropenia occurred less frequently among both Asian patients [2% (3/133)] and white patients [1% (3/226)] administered placebo + H + D.
Discussion
This exploratory analysis of the CLEOPATRA, NeoSphere, and TRYPHAENA studies showed that diarrhea was common in patients receiving pertuzumab-based treatment in either the metastatic or early-stage breast cancer settings. However, most diarrheal episodes were low grade and decreased in frequency with successive treatment cycles. Across the three studies, there was only one grade >3 diarrheal event, a grade 4 event in the control (non-pertuzumab-containing) arm of CLEOPATRA. Sixty-eight percent of patients receiving pertuzumab-based treatment in CLEOPATRA experienced all-grade diarrhea compared with 49% in the control arm; the corresponding values for grade 3 diarrhea were 9% and 5%, respectively. In NeoSphere, both all-grade and grade 3 diarrheal events occurred at the highest rates in patients treated with both pertuzumab and docetaxel. Across all studies, the highest rate of diarrhea was observed with the TCH + P regimen (all grade, 72%; grade 3, 12%) in TRYPHAENA. The lowest rate was seen with the P + H regimen (all grade, 28%; grade 3, 0%) in NeoSphere; of note, this was the only regimen in the present analysis that lacked docetaxel. Additionally, in the subgroup of CLEOPATRA study participants who discontinued docetaxel but continued targeted treatment (P + H or placebo + H), the average diarrheal incidence per treatment cycle was higher during the docetaxel treatment period than the docetaxel-free treatment period. Study drug discontinuations due to diarrhea alone were uncommon, occurring in 0.5%–2% of patients with metastatic breast cancer and in no patient with early-stage breast cancer.
Prior subgroup analyses of CLEOPATRA suggested that the elderly (≥65 years) and patients from Asia with metastatic breast cancer experienced a higher incidence of AEs, including diarrhea [10, 11]. In both patient subgroups, however, the efficacy benefits conferred by P + H + D were found to offset the increased risk of AEs, which were largely managed by docetaxel dose adjustments [10, 11]. In the present analysis, which is based on a more recent data cut, the incidence of all-grade diarrhea within each treatment arm was similar in patients ≥65 versus <65 years of age, but P + H + D-treated patients aged ≥65 years had a higher incidence of grade 3 diarrhea and more diarrhea-associated drug discontinuations and dose delays than patients aged <65 years. The present analysis also indicated that Asian patients—irrespective of treatment (P + H + D or placebo + H + D)—appeared to have a greater incidence and severity of diarrhea than white patients, although this was not associated with an increased frequency of pertuzumab discontinuation due to diarrhea.
Overlap between diarrhea and febrile neutropenia was generally infrequent across the three studies, but occurred at higher frequency in the pertuzumab-containing regimens, with the highest incidence seen in the TCH + P arm (11%) of TRYPHAENA and the lowest incidence seen in the P + H arm (0%) of NeoSphere. Among patients with metastatic breast cancer, the incidence of concurrent diarrhea and febrile neutropenia did not increase with age, but was more pronounced in Asian patients versus white patients. Similarly, no relationship between pre-existing gastrointestinal comorbidities and diarrhea was seen in any of the three studies.
An effective strategy to manage diarrhea should entail proactive follow-up and early intervention with antidiarrheal medications, dietary modifications, rehydration, dose delays, or reductions of the chemotherapy partner. Based on subgroup analyses of CLEOPATRA, patients with metastatic breast cancer aged ≥65 years or those from Asia/Asian patients treated with pertuzumab may require vigilant observation due to their increased susceptibility to grade 3 diarrhea. National Comprehensive Cancer Network guidelines recommend aggressive rehydration and pharmacological intervention for treatment-associated diarrhea in elderly patients [12]. Although these guidelines were developed based on chemotherapy-related diarrhea, a similar approach may be effective for those who experience diarrhea due to the addition of pertuzumab. General adherence to standard practice and published guidelines for treating anti-EGFR-associated diarrhea is also recommended [4, 13]. While the incidence of diarrhea was highest during the first pertuzumab cycle across all study arms, it is important to note that dose reductions of pertuzumab were not permitted in any study and are not advised as a method to treat pertuzumab-associated diarrhea. The indicated loading dose (840 mg) is required to rapidly achieve an effective steady-state concentration, and subsequent fixed dosages are needed to maintain target concentration [14].
In this analysis, diarrhea was frequently treated with loperamide, an opioid commonly prescribed for diarrhea resulting from chemotherapy. However, the efficacy of loperamide in treating pertuzumab-associated diarrhea is unknown. While chemotherapy-induced diarrhea is believed to result from mucosal injury, diarrhea associated with HER2/EGFR signaling blockade may stem from the dysregulation of ion channels in intestinal epithelial cells, which leads to excess chloride secretion [3]. Patients may therefore benefit from treatments tailored to the etiology of this secretory diarrhea; further investigation is warranted.
Supplementary Material
Acknowledgements
The authors thank Bokai Xia of Genentech, Inc. and Allen Lee of Everest Clinical Research Services, Inc. for their statistical programming contributions. Professional medical writing and editorial assistance were provided by Sabrina Hom, PhD, of CodonMedical, an Ashfield Company, part of UDG Healthcare plc, and were funded by Genentech, Inc.
Funding
This work was supported by National Institutes of Health [P30 CA008748] and Genentech, Inc.
Disclosure
SMS has received consulting fees from Genentech, Inc./F. Hoffmann-La Roche, Pfizer, Lilly, AstraZeneca, and Pieris Pharmaceuticals, and fees for non-CME services from Genentech, Inc./F. Hoffmann-La Roche. Her institution has received research funding from Genentech, Inc./F. Hoffmann-La Roche, Pfizer, Merrimack, and Lilly. AS has received consulting fees from F. Hoffmann-La Roche and Celgene and research funding from Celgene. LG has received consulting fees from Genentech, Inc./F. Hoffmann-La Roche, GlaxoSmithKline, Novartis, Pfizer, Boehringer Ingelheim, Celgene, Onkaido Therapeutics, Taiho Pharmaceutical, and Tiziana Life Sciences. AS, MSB, and BY are salaried employees of Genentech, Inc. and own stock in F. Hoffmann-La Roche. MW-L and SH are salaried employees of and own stock in F. Hoffmann-La Roche. JG has received consulting fees from Worldcare, Inc. and participated in an IIT funded by Genentech, Inc. with drugs supplied by Napo Pharmaceuticals. JC has received consulting fees from Novartis, Celgene, and F. Hoffmann-La Roche and honoraria from Novartis, Celgene, F. Hoffmann-La Roche, and Eisai. JB has declared no conflicts of interest.
Footnotes
Note: This study was previously presented in part at San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 8–12 December 2015 (abstr P4-14-14).
References
- 1. Franklin MC, Carey KD, Vajdos FF. et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004; 5: 317–328. [DOI] [PubMed] [Google Scholar]
- 2. Agus DB, Akita RW, Fox WD. et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2: 127–137. [DOI] [PubMed] [Google Scholar]
- 3. Van Sebille YZ, Gibson RJ, Wardill HR, Bowen JM.. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: chloride secretion as a mechanistic hypothesis. Cancer Treat Rev 2015; 41: 646–652. [DOI] [PubMed] [Google Scholar]
- 4. Melosky B. Supportive care treatments for toxicities of anti-egfr and other targeted agents. Curr Oncol 2012; 19(Suppl 1): S59–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson RJ, Keefe DM.. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer 2006; 14: 890–900. [DOI] [PubMed] [Google Scholar]
- 6. Baselga J, Cortés J, Kim SB. et al. ; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012; 366: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swain SM, Baselga J, Kim SB. et al. ; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372: 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gianni L, Pienkowski T, Im YH. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 25–32. [DOI] [PubMed] [Google Scholar]
- 9. Schneeweiss A, Chia S, Hickish T. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013; 24: 2278–2284. [DOI] [PubMed] [Google Scholar]
- 10. Miles D, Baselga J, Amadori D. et al. Treatment of older patients with HER2-positive metastatic breast cancer with pertuzumab, trastuzumab, and docetaxel: subgroup analyses from a randomized, double-blind, placebo-controlled phase III trial (CLEOPATRA). Breast Cancer Res Treat 2013; 142: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swain SM, Im YH, Im SA. et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist 2014; 19: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network. Older Adult Oncology (Version 2.2016). https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf (8 February 2017, date last accessed).
- 13. Califano R, Tariq N, Compton S. et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs 2015; 75: 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng CM, Lum BL, Gimenez V. et al. Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis. Pharm Res 2006; 23: 1275–1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.